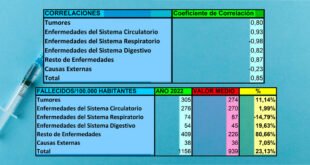
Vacunas, ingredientes
Algunas vacunas tienen antígenos con una cantidad de gérmenes muertos o débiles para que el sistema inmunitario reaccione a las infecciones. El virus de la influenza es un ejemplo de un antígeno.
Los adyuvantes, que se encuentran en algunas vacunas, son sustancias para que el sistema inmunitario reaccione a la vacuna. El aluminio es un ejemplo de un coadyuvante.
Ingredientes tóxicos y mutagénicos en las vacunas infantiles https://www.bitchute.com/video/ilkrSxY0xMke/ , Descargar lista con ingredientes ww.oregon.gov/pharmacy/Documents/excipient-table-2.pdf
Los conservantes, como el timerosal (mercurio), solo testeado en humanos en 1929 y todos los paciente del test murieron. En la actualidad, este conservante se utilizan en viales (envases) de vacunas que tienen más de 1 dosis. Esto se debe a que cada vez que se extrae una dosis individual del vial, es posible que ingresen gérmenes nocivos.
La mayoría de las vacunas también están disponibles en viales de dosis única y contienen cantidades por encima del limite permisible, que quedan del proceso de elaboración pero esto se minimiza.
Hay además estabilizadores, como la gelatina, Material de cultivo (crecimiento) celular, como los huevos, para ayudar a desarrollar antígenos en las vacunas, células madres de fetos humanos abortados.
Ingredientes inactivos (antisépticos), como el formaldehído, para debilitar o eliminar virus, bacterias o toxinas en la vacuna, muy tóxicos y cancerigenos.
Antibióticos, como la neomicina, ayudan a evitar que los gérmenes y bacterias externos se desarrollen en la vacuna.
La FDA (La Administración de Medicamentos y Alimentos de EEUU) ha publicado el Código de Regulaciones Federales, Título 21, Volumen 4, con la máxima tolerancia de aluminio parenteral que es 25 microgramos (mcg) por kg de peso/dia. Parenteral significa cualquier producto que no se administra por vía oral, por lo tanto, las vacunas son consideradas parental. Este documento a continuación aclara, que las personas con problemas en los riñones y bebés prematuros no deben exceder la dosis parenteral de aluminio de 5 microgramos por kilogramo de peso, por día ya que los estudios muestran que el aluminio es tóxico para el sistema nervioso central y a los huesos.
Por lo tanto, si el bebé es más o menos de 4 kg, y el máximo permisible es de 5 mcg x kilo / día, entonces el límite, en este caso, debe ser de 20 microgramos. La vacuna contra la hepatitis B tiene 250 mcg. de aluminio, es decir, que al nacer, sólo con esta dosis, la cantidad de aluminio que se inyecta al recién nacido, es 12 veces mayor de lo que permite la FDA.
Luego en el segundo mes, se inyectan otros 705 mcg. de aluminio (DTaP, Hib, PCV, IPV) cuando la máxima permisible para no tener daño neurológico y óseo es de 30 mcg, es decir, 23 veces los niveles permisibles. Según el sitio web de los CDC, las tasas de autismo son una epidemia, 1 cada 68 niños en los EEUU padece algún tipo dentro del espectro de autismo . Los medios de comunicación generan una alarma exagerada sobre algunos casos de sarampión o paperas , cuando en muchas oportunidades es causado por el contagio de los vacunados, pero nunca tocan el tema de la epidemia de autismo, con toda la evidencia del fraude de los CDC de acuerdo el caso William Thompson .
424 Exipientes de las diferentes vacunas
| EXCIPIENTE | PURPOSE | VACCINE NAME | VACCINE TYPE | AMOUNT PER DOSE |
|---|---|---|---|---|
| 2-Phenoxyethanol | Stabilizer | Adacel | Tdap | 3.3 mg (0.6% v/v) (not as a preservative) |
| 2-Phenoxyethanol | Stabilizer | Daptacel | DTaP | 3.3 mg (0.6% v/v) (not as a preservative) |
| 2-Phenoxyethanol | Preservative | IPOL | Polio | 0.50% |
| 2-Phenoxyethanol | Stabilizer | Pentacel | DTaP+IPV+Hib | 3.3 mg (0.6 v/v) (not as a preservative) |
| 2-Phenoxthanol | Stabilizer | Quadracel | DTaP+IPV | 3.3 mg (0.6 v/v) (not as a preservative) |
| Albumin, Bovine | Stabilizer | Vaqta | HepA | <10–4 mcg |
| Albumin, Bovine Serum | Stabilizer | Ixiaro | Japanese Encephalitis | ≤100 ng/mL |
| Albumin, Bovine Serum | Stabilizer | Pentacel | DTaP+IPV+Hib | ≤50 ng |
| Albumin, Bovine Serum | Stabilizer | Quadracel | DTaP+IPV | ≤50 ng |
| Albumin, Bovine Serum | Stabilizer | RabAvert | Rabies | “Small quantities’, US origin |
| Albumin, Calf Serum | Stabilizer | Kinrix | DTaP+IPV | VERO cell culture growth |
| Albumin, Calf Serum | Stabilizer | Pediarix | DTaP+HepB+IPV | VERO cell culture growth |
| Albumin, Calf Serum | Stabilizer | Zostavax | Zoster | Trace amounts |
| Albumin, Calf Serum Protein | Stabilizer | IPOL | IPV | <50 ng |
| Albumin, Egg (Ovalbumin) | Residual Medium | Afluria | Influenza | <1 mcg |
| Albumin, Egg (Ovalbumin) | Residual Medium | Afluria Quad | Influenza | <1 mcg |
| Albumin, Egg (Ovalbumin) | Residual Medium | Fluarix Quad | Influenza | ≤0.050 mcg |
| Albumin, Egg (Ovalbumin) | Residual Medium | Flulaval Quad | Influenza | ≤0.3 mcg |
| Albumin, Egg (Ovalbumin) | Residual Medium | FluMist Quad | Influenza | ≤0.24 mcg |
| Albumin, Egg (Ovalbumin) | Residual Medium | Fluvirin | Influenza | ≤1 mcg |
| Albumin, Egg (Ovalbumin) | Residual Medium | RabAvert | Rabies | ≤3 ng |
| Albumin, Fetal Bovine Serum | Stabilizer | RotaTeq | Rotavirus | Trace amounts |
| Albumin, Fetal Bovine Serum | Stabilizer | Varivax | Varicella | Trace amounts |
| Albumin, Human | Stabilizer | Imovax | Rabies | <100 mg |
| Albumin, Human | Diluent/Stabilizer | MMR-II | MMR | ≤0.3 mg |
| Albumin, Human | Diluent/Stabilizer | ProQuad | MMRV | 0.31 mg |
| Albumin, Human Serum | Diluent/Stabilizer | RabAvert | Rabies | ≤0.3 mg |
| Aluminum | Adjuvant | Adacel | Tdap | 0.33 mg |
| Aluminum | Adjuvant | Bexsero | Meningococcal Group B | 0.519 mg |
| Aluminum | Adjuvant | BioThrax | Anthrax | 1.2 mg/mL |
| Aluminum | Adjuvant | Boostrix | Tdap | ≤0.39 mg |
| Aluminum | Adjuvant | Daptacel | DTaP | 0.33 mg |
| Aluminum | Adjuvant | DT | DT | 1.5 mg aluminum phosphate |
| Aluminum | Adjuvant | Engerix-B | HepB | 0.5 mg/mL |
| Aluminum | Adjuvant | Gardasil | HPV | 225 mcg |
| Aluminum | Adjuvant | Gardasil 9 | HPV | 500 mcg |
| Aluminum | Adjuvant | Havrix | HepA | 0.5 mg/mL |
| Aluminum | Adjuvant | Infanrix | DTaP | ≤0.625 mg |
| Aluminum | Adjuvant | Ixiaro | Japanese Encephalitis | 250 mcg aluminum hydroxide |
| Aluminum | Adjuvant | Kinrix | DTaP+IPV | ≤0.6 mg |
| Aluminum | Adjuvant | Pediarix | DTaP+HepB+IPV | ≤0.85 mg |
| Aluminum | Adjuvant | PedvaxHIB | Hib+HepB | 225 mcg |
| Aluminum | Adjuvant | Pentacel | DTaP+IPV+Hib | 0.33 mg |
| Aluminum | Adjuvant | Prevnar 13 | Pneumococcal 13-valent | 0.125 mcg |
| Aluminum | Adjuvant | Quadracel | DTaP+IPV | 0.33 mg |
| Aluminum | Adjuvant | Recombivax HB | HepB | 0.5 mg/mL |
| Aluminum | Adjuvant | Td (generic) | Td | ≤0.53 mg |
| Aluminum | Adjuvant | Tenivac | Td | 0.33 mg |
| Aluminum | Adjuvant | Trumenba | Meningococcal Group B | 0.25 mg |
| Aluminum | Adjuvant | Twinrix | HepA+HepB | 0.45 mg |
| Aluminum | Adjuvant | Vaqta | HepA | 0.45 mg |
| Amino Acid | Medium Nutrient | BioThrax | Anthrax | Unspecified amount in growth medium |
| Amino Acid | Medium Nutrient | Flublok | Influenza | Ingredient in growth medium |
| Amino Acid | Medium Nutrient | Flublok Quad | Influenza | Ingredient in growth medium |
| Amino Acid | Medium Nutrient | Gardasil | HPV | Ingredient in growth medium |
| Amino Acid | Medium Nutrient | Gardasil 9 | HPV | Ingredient in growth medium |
| Amino Acid | Medium Nutrient | Havrix | HepA | Amino acid supplement – 0.3% w/v |
| Amino Acid | Medium Nutrient | Menveo | Meningococcal | Ingredient in growth medium |
| Amino Acid | Medium Nutrient | MMR-II | MMR | Ingredient in growth medium |
| Amino Acid | Medium Nutrient | PedvaxHIB | Hib+HepB | Amount not specified in Package Insert |
| Amino Acid | Medium Nutrient | Recombivax HB | HepB | In fermentation medium |
| Amino Acid | Medium Nutrient | Rotarix | Rotavirus | In DMEM (Dulbecco’s Modified Eagle Medium) |
| Amino Acid | Medium Nutrient | Shingrix | Zoster | Ingredient in growth medium |
| Amino Acid | Medium Nutrient | Twinrix | HepA+HepB | Amount not specified in Package Insert |
| Amino Acid | Medium Nutrient | Vivotif | Typhoid | 1.4-7 mg/capsule |
| Ammonium Sulfate | Protein Purifier | ActHIB | Hib | Ingredient in purification |
| Ammonium Sulfate | Protein Purifier | Adacel | Tdap | Ingredient in purification |
| Ammonium Sulfate | Protein Purifier | Daptacel | DTaP | Ingredient in purification |
| Ammonium Sulfate | Protein Purifier | Menactra | Meningococcal | Ingredient in purification |
| Ammonium Sulfate | Protein Purifier | Pentacel | DTaP+IPV+Hib | Ingredient in purification |
| Ammonium Sulfate | Protein Purifier | Prevnar 13 | Pneumococcal 13-valent | Ingredient in purification |
| Ammonium Sulfate | Protein Purifier | Quadracel | DTaP+IPV | Ingredient in purification |
| Ammonium Sulfate | Protein Purifier | Td (generic) | Td | Ingredient in purification |
| Ammonium Sulfate | Protein Purifier | Tenivac | Td | Ingredient in purification |
| Amphotericin B | Antimicrobial | RabAvert | Rabies | ≤20 ng |
| Ascorbic Acid | Antioxidant | Vivotif | Typhoid | 1-5 mg/capsule |
| Barium | Fluad | Influenza | <0.5 mcg | |
| Benzethonium Chloride | Preservative | BioThrax | Anthrax | 25 mcg/mL |
| Beta-Propiolactone | Viral Inactivator | Afluria | Influenza | ≤2 ng |
| Beta-Propiolactone | Viral Inactivator | Afluria Quad | Influenza | ≤1.5 ng |
| Beta-Propiolactone | Viral Inactivator | Flucelvax Quad | Influenza | <0.5 mcg |
| Beta-Propiolactone | Viral Inactivator | Fluvirin | Influenza | ≤0.5 mcg |
| Beta-Propiolactone | Viral Inactivator | Imovax | Rabies | <50 ppm |
| Beta-Propiolactone | Viral Inactivator | RabAvert | Rabies | Amount not specified |
| Bovine Casein | Medium Nutrient | ActHIB | Hib | Amount not specified in Package Insert |
| Bovine Casein | Medium Nutrient | Boostrix | Tdap | Amount not specified in Package Insert |
| Bovine Casein | Medium Nutrient | DT | DT | Amount not specified in Package Insert |
| Bovine Casein | Medium Nutrient | Infanrix | DTaP | Amount not specified in Package Insert |
| Bovine Casein | Medium Nutrient | Kinrix | DTaP+IPV | Amount not specified in Package Insert |
| Bovine Casein | Medium Nutrient | Menactra | Meningococcal | Amount not specified in Package Insert |
| Bovine Casein | Medium Nutrient | Pediarix | DTaP+HepB+IPV | Amount not specified in Package Insert |
| Bovine Casein | Medium Nutrient | Typhim Vi | Typhoid | Amount not specified in Package Insert |
| Bovine Casein | Medium Nutrient | Vivotif | Typhoid | Amount not specified in Package Insert |
| Bovine Extract | Medium Nutrient | Boostrix | Tdap | Amount not specified in Package Insert |
| Bovine Extract | Stabilizer | Infanrix | DTaP | Amount not specified in Package Insert |
| Bovine Extract | Stabilizer | Kinrix | DTaP+IPV | Amount not specified in Package Insert |
| Bovine Extract | Stabilizer | Pediarix | DTaP+HepB+IPV | Amount not specified in Package Insert |
| Bovine Extract | Stabilizer | Td (generic) | Td | Amount not specified in Package Insert |
| Bovine, Casamino Acid | Stabilizer | Adacel | Tdap | Amount not specified in Package Insert |
| Bovine, Casamino Acid | Stabilizer | Daptacel | DTaP | Amount not specified in Package Insert |
| Bovine, Casamino Acid | Stabilizer | Menactra | Meningococcal | Amount not specified in Package Insert |
| Bovine, Casamino Acid | Stabilizer | Menomune | Meningococcal | Amount not specified in Package Insert |
| Bovine, Casamino Acid | Stabilizer | Pentacel | DTaP+IPV+Hib | Amount not specified in Package Insert |
| Bovine, Casamino Acid | Stabilizer | Prevnar 13 | Pneumococcal 13-valent | Amount not specified in Package Insert |
| Bovine, Casamino Acid | Stabilizer | Quadracel | DTaP+IPV | Amount not specified in Package Insert |
| Bovine, Casamino Acid | Stabilizer | Tenivac | Td | Amount not specified in Package Insert |
| Calcium Carbonate | Buffer | Rotarix | Rotavirus | Amount not specified in Package Insert |
| Calcium Chloride | Medium Nutrient | Afluria | Influenza | 0.5 mcg |
| Calcium Chloride | Medium Nutrient | Afluria Quad | Influenza | 0.5 mcg |
| Calcium Chloride | Medium Nutrient | Rotarix | Rotavirus | Amount not specified in Package Insert |
| Carbohydrates | Medium Nutrient | Gardasil | HPV | Amount not specified in Package Insert |
| Carbohydrates | Medium Nutrient | Gardasil 9 | HPV | Amount not specified in Package Insert |
| Chick Embryo | Residual Medium | MMR-II | MMR | No intact cells (may have trace residual protein) |
| Chick Embryo | Residual Medium | ProQuad | MMRV | No intact cells (may have trace residual protein) |
| Chick Embryo | Residual Medium | RabAvert | Rabies | No intact cells (may have trace residual protein) |
| Chick Embryo | Residual Medium | YF-Vax | Yellow Fever | Growth medium |
| Chick fibroblasts | Protein Purifier | RabAvert | Rabies | Growth medium |
| Chlortetracycline | Antimicrobial | RabAvert | Rabies | ≤200 ng |
| Citric Acid Monohydrate | Fluad | Influenza | 0.04 mg | |
| CTAB | Protein Purifier | Fluad | Influenza | ≤12 mcg |
| CTAB | Protein Purifier | Flucelvax Quad | Influenza | ≤18 mcg |
| D-glucose | Medium Nutrient | Rotarix | Rotavirus | In DMEM (Dulbecco’s Modified Eagle Medium) |
| Dextrose | Medium Nutrient | DT | DT | Amount not specified in Package Insert |
| Dextrose | Medium Nutrient | Recombivax HB | HepB | Ingredient in fermentation medium |
| Dextrose | Medium Nutrient | Vivotif | Typhoid | Ingredient in medium |
| Dimethyl-beta-cyclodextrin | Medium Nutrient | Adacel | Tdap | Amount not specified in Package Insert |
| Dimethyl-beta-cyclodextrin | Medium Nutrient | Daptacel | DTaP | Amount not specified in Package Insert |
| Dimethyl-beta-cyclodextrin | Medium Nutrient | Pentacel | DTaP+IPV+Hib | Ingredient in Stainer-Scholte medium |
| DNA | Residual Medium | Flublok | Influenza | ≤10 ng |
| DNA | Residual Medium | Flublok Quad | Influenza | ≤10 ng |
| DNA | Residual Medium | Flucelvax Quad | Influenza | ≤10 ng MDCK cell |
| DNA | Residual Medium | Ixiaro | Japanese Encephalitis | ≤200 pg/mL (‘host cell DNA’) |
| DNA | Residual Medium | ProQuad | MMRV | Residual component of MRC-5 cells |
| DNA | Residual Medium | RotaTeq | Rotavirus | Residual component of manufacturing process |
| DNA | Residual Medium | Shingrix | Zoster | ≤2.1 pg |
| DNA | Residual Medium | Vaqta | HepA | <4 x 10–6 mcg |
| DNA | Residual Medium | Varivax | Varicella | Residual component of MRC-5 cells |
| DNA | Residual Medium | Zostavax | Zoster | Residual component of MRC-5 cells |
| DOPC | Adjuvant | Shingrix | Zoster | 1 mg |
| EDTA | Preservative | FluMist Quad | Influenza | <0.37 mcg |
| EDTA | Preservative | RabAvert | Rabies | 0.3 mg sodium EDTA |
| EDTA | Preservative | Varivax | Varicella | Trace amounts |
| Egg Protein | Residual Medium | Fluad | Influenza | <0.4 mcg |
| Egg Protein | Residual Medium | Fluvirin | Influenza | ≤1 mcg ovalbumin |
| Ferric (III) Nitrate | Medium Nutrient | Rotarix | Rotavirus | In DMEM (Dulbecco’s Modified Eagle Medium) |
| Formaldehyde | Inactivating Agent | ActHIB | Hib | <0.5 mcg |
| Formaldehyde | Inactivating Agent | Adacel | Tdap | ≤5 mcg |
| Formaldehyde | Preservative | BioThrax | Anthrax | 100 mcg/mL |
| Formaldehyde | Inactivating Agent | Boostrix | Tdap | ≤100 mcg (residual) |
| Formaldehyde | Inactivating Agent | Daptacel | DTaP | ≤5 mcg (residual) |
| Formaldehyde | Inactivating Agent | DT | DT | <100 mcg |
| Formaldehyde | Inactivating Agent | Fluad | Influenza | ≤10 mcg |
| Formaldehyde | Inactivating Agent | Fluarix Quad | Influenza | ≤5 mcg |
| Formaldehyde | Inactivating Agent | Flulaval Quad | Influenza | ≤25 mcg |
| Formaldehyde | Inactivating Agent | FluZone High-Dose | Influenza | ≤100 mcg |
| Formaldehyde | Inactivating Agent | FluZone Id Quad | Influenza | ≤20 mcg |
| Formaldehyde | Inactivating Agent | FluZone Quad | Influenza | ≤100 mcg |
| Formaldehyde | Inactivating Agent | Hiberix | Hib | <0.5 mcg (residual) |
| Formaldehyde | Inactivating Agent | Infanrix | DTaP | ≤100 mcg (residual) |
| Formaldehyde | Inactivating Agent | IPOL | Polio | ≤0.02% |
| Formaldehyde | Inactivating Agent | Ixiaro | Japanese Encephalitis | ≤200 pmm |
| Formaldehyde | Inactivating Agent | JE-Vax | Japanese Encephalitis | <100 mcg |
| Formaldehyde | Inactivating Agent | Kinrix | DTaP+IPV | ≤100 mcg (residual) |
| Formaldehyde | Inactivating Agent | Menactra | Meningococcal | <2.66 mcg |
| Formaldehyde | Inactivating Agent | Menveo | Meningococcal | ≤0.3 mcg |
| Formaldehyde | Inactivating Agent | Pediarix | DTaP+HepB+IPV | ≤100 mcg (residual) |
| Formaldehyde | Inactivating Agent | Pentacel | DTaP+IPV+Hib | ≤5 mcg (residual) |
| Formaldehyde | Inactivating Agent | Quadracel | DTaP+IPV | ≤5 mcg |
| Formaldehyde | Inactivating Agent | Recombivax HB | HepB | <15 mcg/mL (residual) |
| Formaldehyde | Inactivating Agent | Td (generic) | Td | 100 mcg |
| Formaldehyde | Inactivating Agent | Tenivac | Td | ≤5 mcg (residual) |
| Formaldehyde | Inactivating Agent | Twinrix | HepA+HepB | ≤0.1 mg formalin |
| Formaldehyde | Inactivating Agent | Typhim Vi | Typhoid | ≤100 mcg (residual) |
| Formaldehyde | Inactivating Agent | Vaqta | HepA | <0.8 mcg |
| Galactose | Medium Nutrient | Vivotif | Typhoid | Ingredient in medium |
| Gelatin | Manufacturing Residue | JE-Vax | Japanese Encephalitis | 500 mcg/1 mL dose |
| Gelatin | Gelatin Capsules | Vivotif | Typhoid | Gelatin capsules |
| Gelatin | Stabilizer | YF-Vax | Yellow Fever | Amount not specified in Package Insert |
| Gelatin, Bovine | Stabilizer, solvent | RabAvert | Rabies | ≤12 mg polygeline |
| Gelatin, hydrolyzed | Stabilizer | MMR-II | MMR | 14.5 mg |
| Gelatin, hydrolyzed | Stabilizer | ProQuad | MMRV | 11 mg |
| Gelatin, hydrolyzed | Stabilizer | Varivax | Varicella | 12.5 mg |
| Gelatin, Porcine, Hydrolyzed | Stabilizer | FluMist Quad | Influenza | 2.00 mg/0.2 mLdose |
| Gelatin, Porcine, Hydrolyzed | Stabilizer | Zostavax | Varicella Zoster | 15.58 mg |
| Gentamicin Sulfate | Antimicrobial | Fluarix Quad | Influenza | ≤0.15 mcg |
| Gentamicin Sulfate | Antimicrobial | FluMist Quad | Influenza | <0.015 mcg/mL |
| Glutamate | Medium Nutrient | MMR-II | MMR | Ingredient in growth medium (Medium 199) |
| Glutaraldehyde | Inactivating Agent | Adacel | Tdap | <50 ng (residual) |
| Glutaraldehyde | Inactivating Agent | Boostrix | Tdap | Amount not specified in Package Insert |
| Glutaraldehyde | Inactivating Agent | Daptacel | DTaP | <50 ng (residual) |
| Glutaraldehyde | Inactivating Agent | Infanrix | DTaP | Amount not specified in Package Insert |
| Glutaraldehyde | Inactivating Agent | Kinrix | DTaP+IPV | Amount not specified in Package Insert |
| Glutaraldehyde | Inactivating Agent | Pediarix | DTaP+HepB+IPV | Amount not specified in Package Insert |
| Glutaraldehyde | Inactivating Agent | Pentacel | DTaP+IPV+Hib | <50 ng (residual) |
| Glutaraldehyde | Inactivating Agent | Quadracel | DTaP+IPV | <50 ng |
| Hexadecyltrimethylammonium Bromide | Protein Purifier | Typhim Vi | Typhoid | Amount not specified in Package Insert |
| Histidine | Medium Nutrient | Bexsero | Meningococcal Group B | 0.776 mg |
| Histidine | Medium Nutrient | Trumenba | Meningococcal Group B | 10 mM |
| Hydrocortisone | Medium Nutrient | Fluarix Quad | Influenza | ≤0.0016 mcg |
| Kanamycin | Antimicrobial | Bexero | Meningococcal | 0.01 mcg |
| Kanamycin | Antimicrobial | Fluad | Influenza | ≤0.01 mcg |
| L-cystine | Medium Nutrient | Rotarix | Rotavirus | Amount not specified in Package Insert |
| L-histidine | Medium Nutrient | Gardasil | HPV | 0.78 mg |
| L-histidine | Medium Nutrient | Gardasil 9 | HPV | 0.78 mg |
| L-tyrosine | Medium Nutrient | Rotarix | Rotavirus | Amount not specified in Package Insert |
| Lactalbumin Hydrolysate | Medium Nutrient | Kinrix | DTaP+IPV | Ingredient in growth medium |
| Lactalbumin Hydrolysate | Medium Nutrient | Pediarix | DTaP+HepB+IPV | Ingredient in growth medium |
| Lactose | Stabilizer | BCG Vaccine | BCG | Amount not specified in Package Insert |
| Lactose | Stabilizer | Hiberix | Hib | 12.6 mg |
| Lactose | Stabilizer | Menomune | Meningococcal | 2.5-5 mg |
| Lactose | Stabilizer | Vivotif | Typhoid | 100-180 mg/capsule |
| Magnesium Stearate | Lubricant for capsule filling | Vivotif | Typhoid | 3.6-4.4 mg/capsule |
| Magnesium Sulfate | Medium Nutrient | Rotarix | Rotavirus | Amount not specified in Package Insert |
| Monosodium glutamate | Stabilizer | FluMist Quad | Influenza | 0.188 mg/0.2 mL dose |
| Monosodium L-glutamate | Stabilizer | ProQuad | MMRV | 0.40 mg |
| Monosodium L-glutamate | Stabilizer | Varivax | Varicella | 0.5 mg |
| Monosodium L-glutamate | Stabilizer | Zostavax | Varicella Zoster | 0.62 mg |
| Mouse Serum Protein | Manufacturing Residue | JE-Vax | Japanese Encephalitis | <50 ng |
| MRC-5 | Manufacturing Residue | Havrix | HepA | ≤5 mcg/mL |
| MRC-5 | Manufacturing Residue | Imovax | Rabies | Amount not specified in Package Insert |
| MRC-5 | Growth Medium | Pentacel | DTaP+IPV+Hib | Amount not specified in Package Insert |
| MRC-5 | Manufacturing Residue | ProQuad | MMRV | Residual |
| MRC-5 | Manufacturing Residue | Quadracel | DTaP+IPV | Ingredient in growth medium |
| MRC-5 | Manufacturing Residue | Twinrix | HepA+HepB | ≤2.5 mcg |
| MRC-5 | Manufacturing Residue | Vaqta | HepA | Amount not specified in Package Insert |
| MRC-5 | Manufacturing Residue | Varivax | Varicella | Residual components |
| MRC-5 | Manufacturing Residue | Zostavax | Varicella Zoster | Residual |
| Neomycin | Antimicrobial | Fluad | Influenza | ≤0.02 mcg |
| Neomycin | Antimicrobial | Fluvirin | Influenza | ≤2.5 mcg |
| Neomycin | Antimicrobial | Havrix | HepA | ≤40 ng |
| Neomycin | Antimicrobial | IPOL | IPV | <5 ng |
| Neomycin | Antimicrobial | Kinrix | DTaP+IPV | ≤0.05 ng |
| Neomycin | Antimicrobial | MMR-II | MMR | 25 mcg |
| Neomycin | Antimicrobial | Pediarix | DTaP+HepB+IPV | ≤0.05 ng |
| Neomycin | Antimicrobial | Pentacel | DTaP+IPV+Hib | <4 pg |
| Neomycin | Antimicrobial | ProQuad | MMRV | <16 mcg |
| Neomycin | Antimicrobial | Quadracel | DTaP+IPV | <4 pg |
| Neomycin | Antimicrobial | RabAvert | Rabies | <10 mcg |
| Neomycin | Antimicrobial | Vaqta | HepA | <10 ppb (residual) |
| Neomycin | Antimicrobial | Varivax | Varicella | Trace amounts |
| Neomycin | Antimicrobial | Zostavax | Varicella Zoster | Trace amounts |
| Neomycin Sulfate | Antimicrobial | Afluria | Influenza | ≤61.5 ng |
| Neomycin Sulfate | Antimicrobial | Afluria Quad | Influenza | ≤81.8 ng |
| Neomycin Sulfate | Antimicrobial | Imovax | Rabies | <150 mcg |
| Neomycin Sulfate | Antimicrobial | Twinrix | HepA+HepB | ≤20 ng |
| Nonylphenol Ethoxylate | Surfactant | Fluvirin | Influenza | ≤0.015% w/v |
| Octoxynol-10 (Triton X-100) | Surfactant | Fluarix Quad | Influenza | ≤0.115 mg |
| Octoxynol-10 (Triton X-100) | Surfactant | Flublok | Influenza | ≤100 mcg |
| Octoxynol-10 (Triton X-100) | Surfactant | Flublok Quad | Influenza | ≤100 mcg |
| Octylphenol Ethoxylate (Triton X-100) | Surfactant | FluZone High-Dose | Influenza | ≤250 mcg |
| Octylphenol Ethoxylate (Triton X-100) | Surfactant | FluZone Intradermal Quad | Influenza | ≤55 mcg |
| Octylphenol Ethoxylate (Triton X-100) | Surfactant | FluZone Quad | Influenza | ≤250 mcg/0.5mL |
| Peptone, Soy | Medium Nutrient | Prevnar 13 | Pneumococcal 13-valent | Growth medium |
| Peptone, Soy | Medium Nutrient | Recombivax HB | HepB | Ingredient in fermentation medium |
| Phenol | Preservative | Pneumovax 23 | Pneumococcal 23-valent | Saline containing 0.25% phenol |
| Phenol | Preservative | Typhim Vi | Typhoid | 0.25% |
| Phenol | Preservative, antibacterial | PedvaxHIB | Hib+HepB | Ingredient in purification procedure |
| Phenol Red Indicator | pH indicator, dye | Imovax | Rabies | 20 mcg |
| Phenol Red Indicator | pH indicator, dye | Rotarix | Rotavirus | Ingredient in growth medium |
| Phosphate Buffer | Buffer | Afluria | Influenza | 20 mcg monobasic potassium phosphate |
| Phosphate Buffer | Buffer | Afluria Quad | Influenza | 20 mcg monobasic potassium phosphate |
| Phosphate Buffer | Buffer | Engerix-B | HepB | 0.98 mg/mL disodium phosphate dihydrate |
| Phosphate Buffer | Buffer | Flublok | Influenza | 0.195 mcg monobasic sodium phosphate |
| Phosphate Buffer | Buffer | Flublok Quad | Influenza | 0.195 mcg monobasic sodium phosphate |
| Phosphate Buffer | Buffer | FluMist Quad | Influenza | 0.96 mg/0.2 mL dose monobasic potassium phosphate |
| Phosphate Buffer | Buffer | FluZone High-Dose | Influenza | Sodium phosphate-buffered isotonic sodium chloride solution – “quantity sufficient to appropriate volume” |
| Phosphate Buffer | Buffer | FluZone Intradermal Quad | Influenza | Sodium phosphate-buffered isotonic sodium chloride solution – “quantity sufficient to appropriate volume” |
| Phosphate Buffer | Buffer | FluZone Quad | Influenza | Sodium phosphate-buffered isotonic sodium chloride solution – “quantity sufficient to appropriate volume” |
| Phosphate Buffer | Buffer | Heplisav-B | HepB | 9.0 mg/mL sodium chloride |
| Phosphate Buffer | Buffer | Ixiaro | Japanese Encephalitis | Potassium dihydrogen phosphate |
| Phosphate Buffer | Buffer | Menactra | Meningococcal | Sodium phosphate buffered isotonic sodium chloride solution, amount not specified |
| Phosphate Buffer | Buffer | MMR-II | MMR | Sodium phosphate, amount not specified |
| Phosphate Buffer | Buffer | ProQuad | MMRV | 36 mcg potassium phosphate dibasic |
| Phosphate Buffer | Buffer | Rotarix | Rotavirus | Sodium phosphate in DMEM (medium) |
| Phosphate Buffer | Buffer | RotaTeq | Rotavirus | Sodium phosphate monobasic monohydrate |
| Phosphate Buffer | Buffer | Shingrix | Zoster | 0.54 mg potassium dihydrogen phosphate |
| Phosphate Buffer | Buffer | Twinrix | HepA+HepB | Amount not specified in Package Insert |
| Phosphate Buffer | Buffer | Typhim Vi | Typhoid | 0.065 mg disodium phosphate |
| Phosphate Buffer | Buffer | Varivax | Varicella | 0.08 mg potassium phosphate monobasic |
| Phosphate Buffer | Buffer | Zostavax | Varicella Zoster | 0.10 mg potassium phosphate monobasic |
| Polymyxin | Antimicrobial | Fluvirin | Influenza | ≤3.75 mcg |
| Polymyxin B | Antimicrobial | Afluria | Influenza | ≤10.5 ng |
| Polymyxin B | Antimicrobial | Afluria Quad | Influenza | ≤14 ng |
| Polymyxin B | Antimicrobial | IPOL | Polio | 25 ng |
| Polymyxin B | Antimicrobial | Kinrix | DTaP+IPV | ≤0.01 ng |
| Polymyxin B | Antimicrobial | Pediarix | DTaP+HepB+IPV | ≤0.01 ng |
| Polymyxin B | Antimicrobial | Pentacel | DTaP+IPV+Hib | <4 pg |
| Polymyxin B sulfate | Antimicrobial | Quadracel | DTaP+IPV | <4 pg |
| Polysorbate 20 | Surfactant | Flublok | Influenza | ≤27.5 mcg (Tween 20) |
| Polysorbate 20 | Surfactant | Flublok Quad | Influenza | ≤27.5 mcg (Tween 20) |
| Polysorbate 20 | Surfactant | Havrix | HepA | 0.05 mg/mL |
| Polysorbate 20 | Surfactant | Twinrix | HepA+HepB | Amount not specified in Package Insert |
| Polysorbate 80 | Surfactant | Boostrix | Tdap | ≤100 mcg (Tween 80) |
| Polysorbate 80 | Surfactant | Fluad | Influenza | 1.175 mg |
| Polysorbate 80 | Surfactant | Fluarix Quad | Influenza | ≤0.550 mg (Tween 80) |
| Polysorbate 80 | Surfactant | Flucelvax Quad | Influenza | ≤1500 mcg (Tween 80) |
| Polysorbate 80 | Surfactant | Flulaval Quad | Influenza | ≤887 mcg |
| Polysorbate 80 | Surfactant | Gardasil | HPV | 50 mcg |
| Polysorbate 80 | Surfactant | Gardasil 9 | HPV | 50 mcg |
| Polysorbate 80 | Surfactant | Heplisav-B | HepB | 0.1 mg/mL |
| Polysorbate 80 | Surfactant | Infanrix | DTaP | ≤100 mcg (Tween 80) |
| Polysorbate 80 | Surfactant | JE-Vax | Japanese Encephalitis | <0.0007% |
| Polysorbate 80 | Surfactant | Kinrix | DTaP+IPV | ≤100 mcg (Tween 80) |
| Polysorbate 80 | Surfactant | Pediarix | DTaP+HepB+IPV | ≤100 mcg (Tween 80) |
| Polysorbate 80 | Surfactant | Pentacel | DTaP+IPV+Hib | 10 ppm |
| Polysorbate 80 | Surfactant | Prevnar 13 | Pneumococcal 13-valent | 100 mcg |
| Polysorbate 80 | Surfactant | Quadracel | DTaP+IPV | 10 ppm |
| Polysorbate 80 | Surfactant | RotaTeq | Rotavirus | Amount not specified in Package Insert |
| Polysorbate 80 | Surfactant | Shingrix | Zoster | 0.08 mg |
| Polysorbate 80 | Surfactant | Trumenba | Meningococcal Group B | 0.018 mg |
| Porcine DNA | Residual Medium | Rotarix | Rotavirus | PCV-1 |
| Porcine DNA | Residual Medium | RotaTeq | Rotavirus | PCV-1 and PCV-2 |
| Potassium Aluminum Sulfate | Adjuvant | Recombivax HB | HepB | Amount not specified in Package Insert |
| Potassium Chloride | Buffer | Afluria | Influenza | 20 mcg |
| Potassium Chloride | Buffer | Afluria Quad | Influenza | 20 mcg |
| Potassium Chloride | Buffer | ProQuad | MMRV | 60 mcg |
| Potassium Chloride | Medium Nutrient | Rotarix | Rotavirus | Ingredient in DMEM medium |
| Potassium Chloride | Buffer | Varivax | Varicella | 0.08 mg |
| Potassium Chloride | Buffer | Zostavax | Varicella Zoster | 0.10 mg |
| Potassium Glutamate | Stabilizer | RabAvert | Rabies | 1 mg |
| Protamine Sulfate | Protein Purifier | Ixiaro | Japanese Encephalitis | ≤1 mcg/mL |
| Salts and Sugars, Inorganic | Medium Nutrient | BioThrax | Anthrax | Amount not specified in Package Insert |
| Salts, Inorganic | Medium Nutrient | DT | DT | Sodium chloride |
| Salts, Inorganic | Medium Nutrient | Twinrix | HepA+HepB | Sodium chloride |
| Salts, Mineral | Adjust tonicity | ActHIB | Hib | 0.4% sodium chloride in diluent |
| Salts, Mineral | Adjust tonicity | Afluria | Influenza | 4.1 mg sodium chloride |
| Salts, Mineral | Adjust tonicity | Afluria Quad | Influenza | 4.1 mg sodium chloride |
| Salts, Mineral | Adjust tonicity | Bexsero | Meningococcal Group B | 3.125 mg sodium chloride |
| Salts, Mineral | Adjust tonicity | BioThrax | Anthrax | 0.85% sodium chloride |
| Salts, Mineral | Adjust tonicity | Boostrix | Tdap | 4.4 mg sodium chloride |
| Salts, Mineral | Adjust tonicity | Engerix-B | HepB | 9 mg/mL sodium chloride |
| Salts, Mineral | Adjust tonicity | Flublok | Influenza | 4.4 mg sodium chloride |
| Salts, Mineral | Adjust tonicity | Flublok Quad | Influenza | 4.4 mg sodium chloride |
| Salts, Mineral | Adjust tonicity | Gardasil | HPV | 9.56 mg sodium chloride |
| Salts, Mineral | Adjust tonicity | Gardasil 9 | HPV | 9.56 mg sodium chloride |
| Salts, Mineral | Adjust tonicity | Hiberix | Hib | 0.9% sodium chloride in diluent |
| Salts, Mineral | Adjust tonicity | Infanrix | DTaP | 4.5 mg sodium chloride |
| Salts, Mineral | Adjust tonicity | Kinrix | DTaP+IPV | 4.5 mg sodium chloride |
| Salts, Mineral | Adjust tonicity | MMR-II | MMR | Sodium chloride, amount not specified |
| Salts, Mineral | Adjust tonicity | Pediarix | DTaP+HepB+IPV | 4.5 mg sodium chloride |
| Salts, Mineral | Adjust tonicity | PedvaxHIB | Hib+HepB | 0.9% sodium chloride |
| Salts, Mineral | Adjust tonicity | ProQuad | MMRV | 2.4 mg sodium chloride |
| Salts, Mineral | Adjust tonicity | Recombivax HB | HepB | Ingredient in fermentation medium |
| Salts, Mineral | Adjust tonicity | Rotarix | Rotavirus | In DMEM (Dulbecco’s Modified Eagle Medium) |
| Salts, Mineral | Adjust tonicity | Shingrix | Zoster | 4.385 mg sodium chloride |
| Salts, Mineral | Adjust tonicity | Tenivac | Td | Sodium chloride, amount not specified |
| Salts, Mineral | Adjust tonicity | Twinrix | HepA+HepB | Sodium chloride, amount not specified |
| Salts, Mineral | Adjust tonicity | Typhim Vi | Typhoid | 4.150 mg sodium chloride |
| Salts, Mineral | Adjust tonicity | Vaqta | HepA | 0.9% sodium chloride |
| Salts, Mineral | Adjust tonicity | Varivax | Varicella | 3.2 mg sodium chloride |
| Salts, Mineral | Adjust tonicity | YF-Vax | Yellow Fever | Diluent |
| Salts, Mineral | Adjust tonicity | Zostavax | Varicella Zoster | 3.99 mg sodium chloride |
| Sodium Bicarbonate | Buffer | ProQuad | MMRV | 0.17 mg |
| Sodium Borate | Buffer | Gardasil | HPV | 35 mcg |
| Sodium Borate | Buffer | Gardasil 9 | HPV | 35 mcg |
| Sodium Borate | Stabilizer | Vaqta | HepA | 70 mcg/mL |
| Sodium Citrate | Adjust pH | RotaTeq | Rotavirus | Amount not specified in Package Insert |
| Sodium Citrate Dihydrate | Fluad | Influenza | 0.66 mg | |
| Sodium Deoxycholate | Surfactant | Fluarix Quad | Influenza | ≤65 mcg |
| Sodium Deoxycholate | Surfactant | Flulaval Quad | Influenza | ≤50 mcg |
| Sodium Hydroxide | RotaTeq | Rotavirus | Amount not specified in Package Insert | |
| Sodium Metabisulphite | Stabilizer | Ixiaro | Japanese Encephalitis | ≤200 ppm |
| Sodium Pyruvate | Medium Nutrient | Rotarix | Rotavirus | in DMEM (medium) |
| Sodium Taurodeoxycholate | Protein Purifier | Afluria Quad | Influenza | ≤10 ppm (residual) |
| Sorbitan Trioleate | Fluad | Influenza | 1.175 mg | |
| Sorbitol | Stabilizer, solvent | MMR-II | MMR | 14.5 mg |
| Sorbitol | Stabilizer, solvent | ProQuad | MMRV | 1.8 mg |
| Sorbitol | Stabilizer, solvent | Rotarix | Rotavirus | Amount not specified in Package Insert |
| Sorbitol | Stabilizer, solvent | YF-Vax | Yellow Fever | Amount not specified in Package Insert |
| Streptomycin | Antimicrobial | IPOL | Polio | 200 ng |
| Succinate Buffer | Stabilizer | Prevnar 13 | Pneumococcal 13-valent | 295 mcg |
| Sucrose | Stabilizer | ActHIB | Hib | 8.50% |
| Sucrose | Stabilizer | Afluria | Influenza | <10 mcg |
| Sucrose | Stabilizer | Afluria Quad | Influenza | <10 mcg |
| Sucrose | Stabilizer | Bexsero | Meningococcal Group B | 10 mg |
| Sucrose | Stabilizer | FluMist Quad | Influenza | 13.68 mg/0.2 mL dose |
| Sucrose | Stabilizer | JE-Vax | Japanese Encephalitis | 40% w/v |
| Sucrose | Stabilizer | MMR-II | MMR | 1.9 mg |
| Sucrose | Stabilizer | Pentacel | DTaP+IPV+Hib | 42.5 mg |
| Sucrose | Stabilizer | ProQuad | MMRV | ≤21 mg |
| Sucrose | Stabilizer | Rotarix | Rotavirus | Amount not specified in Package Insert |
| Sucrose | Stabilizer | RotaTeq | Rotavirus | Amount not specified in Package Insert |
| Sucrose | Stabilizer | Shingrix | Zoster | 20 mg |
| Sucrose | Stabilizer | Varivax | Varicella | 25 mg |
| Sucrose | Stabilizer | Vivotif | Typhoid | 3.3-34.2 mg/capsule |
| Sucrose | Stabilizer | Zostavax | Varicella Zoster | 31.16 mg |
| Thimerosal | Preservative | Afluria | Influenza | 24.5 mcg mercury only in multi-dose vial; none in single dose |
| Thimerosal | Preservative | Afluria Quad | Influenza | 24.5 mcg mercury only in multi-dose vial; none in single dose |
| Thimerosal | Preservative | Flucelvax Quad | Influenza | <25 mcg mercury only in multi-dose vial; none in single dose |
| Thimerosal | Preservative | Flulaval Quad | Influenza | <25 mcg mercury only in multi-dose vial; none in single dose |
| Thimerosal | Preservative | Fluvirin | Influenza | 25 mcg mercury/dose in multi-dose vial; ≤1 mcg/dose mercury in prefilled syringe |
| Thimerosal | Preservative | FluZone Quad | Influenza | 25 mcg mercury in multi-dose vial; none in single dose |
| Thimerosal | Preservative | JE-Vax | Japanese Encephalitis | 0.01% |
| Thimerosal | Preservative | Menomune | Meningococcal | 25 mcg mercury/dose in multi-dose diluent; none in single-dose |
| Thimerosal | Manufacturing Residue | Td (generic) | Td | ≤0.3 mcg mercury (trace amounts) |
| Vero Cells | Growth Medium | IPOL | Polio | Amount not specified in Package Insert |
| Vero cells | Medium Nutrient | Ixiaro | Japanese Encephalitis | Amount not specified in Package Insert |
| Vero Cells | Growth Medium | Kinrix | DTaP+IPV | Amount not specified in Package Insert |
| Vero Cells | Growth Medium | Pediarix | DTaP+HepB+IPV | Amount not specified in Package Insert |
| Vero Cells | Growth Medium | Rotarix | Rotavirus | Amount not specified in Package Insert |
| Vero Cells | Growth Medium | RotaTeq | Rotavirus | Amount not specified in Package Insert |
| Vitamins | Medium Nutrient | BioThrax | Anthrax | Ingredient in growth medium |
| Vitamins | Medium Nutrient | DT | DT | Ingredient in growth medium |
| Vitamins | Protein Purifier | Fluarix Quad | Influenza | ≤0.135 mg α-tocopheryl hydrogen succinate |
| Vitamins | Protein Purifier | Flublok | Influenza | Ingredient in growth medium |
| Vitamins | Protein Purifier | Flublok Quad | Influenza | Ingredient in growth medium |
| Vitamins | Protein Purifier | Flulaval Quad | Influenza | ≤320 mcg α-tocopheryl hydrogen succinate |
| Vitamins | Medium Nutrient | Gardasil | HPV | Ingredient in growth medium |
| Vitamins | Medium Nutrient | Gardasil 9 | HPV | Ingredient in growth medium |
| Vitamins | Medium Nutrient | Heplisav-B | HepB | Ingredient in grown media |
| Vitamins | Medium Nutrient | MMR-II | MMR | Ingredient in grown media |
| Vitamins | Medium Nutrient | Rotarix | Rotavirus | Amount not specified in Package Insert |
| Xanthan | Thickening agent | Rotarix | Rotavirus | Ingredient in diluent |
| Yeast | Medium Nutrient | Comvax | Hib+HepB | “contains no detectable yeast DNA, and 1% or less of the protein is of yeast origin” |
| Yeast | Medium Nutrient | Engerix-B | HepB | ≤5% yeast protein |
| Yeast | Medium Nutrient | Gardasil | HPV | <7 mcg yeast protein/dose |
| Yeast | Medium Nutrient | Gardasil 9 | HPV | <7 mcg yeast protein/dose |
| Yeast | Medium Nutrient | Heplisav-B | HepB | Yeast protein: residual amounts (≤5% of total protein) |
| Yeast | Medium Nutrient | Menveo | Meningococcal | Ingredient in growth medium |
| Yeast | Medium Nutrient | Pediarix | DTaP+HepB+IPV | ≤5% yeast protein |
| Yeast | Medium Nutrient | Prevnar 13 | Pneumococcal 13-valent | Ingredient in growth medium |
| Yeast | Medium Nutrient | Recombivax HB | HepB | <1% yeast protein |
| Yeast | Medium Nutrient | Twinrix | HepA+HepB | ≤5% yeast protein |
| Yeast | Medium Nutrient | Vivotif | Typhoid | Amount not specified in Package Insert |
Descargar lista con ingredientes ww.oregon.gov/pharmacy/Documents/excipient-table-2.pdf
Vacunas, Solo con la de Hepatitis B, sobredosis de aluminio
https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?fr=201.323
Combining Childhood Vaccines at One Visit Is Not Safe Neil Z. Miller
http://www.jpands.org/vol21no2/miller.pdf
https://www.cdc.gov/niosh/docs/2005-106/pdfs/2005-106.pdf
https://www.fda.gov/BiologicsBloodVaccines/Vaccines/ApprovedProducts/ucm093833.htm
https://cienciaysaludnatural.com/aluminio
Si encuentra “ este artículo útil suscríbase como miembro de nuestro equipo para apoyarnos y en conjunto podamos aclarar el tema en discusiones con familiares, amigos, funcionarios y empleadores. L Muchas gracias. Puede Apoyarnos en https://cienciaysaludnatural.com/colaboracion
Colabore por favor con nosotros para que podamos incluir mas información y llegar a más personas: contribución en mercado pago o paypal por única vez, Muchas Gracias!
Via PAYPAL: Euros o dólares click aqui
ARGENTINA 10.000$ar https://mpago.la/1srgnEY
5.000$ar https://mpago.la/1qzSyt9
1.000$ar https://mpago.la/1Q1NEKM
Solicite nuestro CBU contactenos