Author: Roxana Bruno, Biochemistry PhD in Immunology.
Striking similarity between human syncytins and the sars-cov-2 spike protein: Why covid-19 vaccines might affect fertility
SUMMARY
COVID-19 vaccines carry the spike protein (S or «Spike») of the SARS-CoV-2 virus as an alleged antigen to trigger the immune response, which shares high genetic and protein similarity with two human proteins, Sincitin-1 and Sincitin-2.
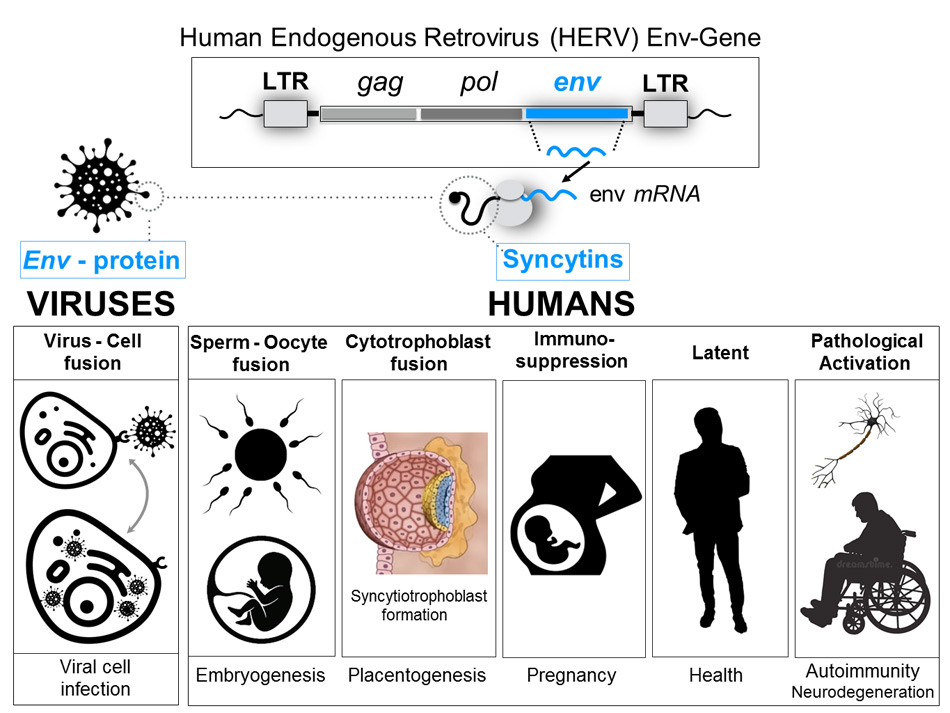
Human syncytins are the product of the expression of the genes of the envelope (Env) of human endogenous retroviruses (HERV): they are proteins that mediate fusion between cells and have immunosuppressive properties.
Syncytins are physiologically expressed during pregnancy: they intervene in the development of the placenta, trophoblast differentiation, the implantation of the embryo in the mother’s uterus and the immunosuppression of the mother’s immune system to prevent allogeneic rejection of the embryo.
Because of the similarity between syncytins and the spike protein of SARS-CoV-2, COVID-19 vaccine-induced antibody responses could trigger a cross-reaction against syncytins, causing allergic, cytotoxic and/or autoimmune side effects affecting human health and reproduction.
mRNA vaccines have the potential to modify human DNA by the mechanism of gene silencing mediated by interference RNA. Syncytin gene could be silenced by using antisense oligonucleotide inhibitors. When the mRNA or the amount of syncytin protein decreases, severe defects in the placenta, poor differentiation of the human trophoblast and placental vascular dysfunction occur, leading to loss of gestation.
The companies developing COVID-19 vaccines are not acting ethically and responsibly, because they do not carry out the safety studies in the appropriate animal models, they are not respecting the times required to detect adverse effects in the medium and long term and, in addition, they are not providing the information about the true vaccine composition, which they consider «confidential».
Volunteers are not being properly informed of all the risks involved in vaccination. By advancing and shortening the experimental phases, companies are shifting the risk from animals to humans, using people as models of animal challenge.
The consequences of inoculating foreign genes with COVID-19 vaccines
may be catastrophic for the fate of mankind, considering the role of HERV envelope proteins (syncytins) in human
physiology and their possible pathogenic effects on various types of cancers
and autoimmune disorders.
The striking similarity between endogenous human retroviral proteins and the SARS-COV-2 spike protein.
Qualified scientific and medical researchers are warning the international community of the danger posed by vaccines against COVID-19 and are calling on the authorities to immediately halt Phase III clinical trials of vaccines containing the spike protein (S or «Spike») mRNA of the SARS-CoV-2 virus 1, 2 .
One of the reasons for this urgent request is based on the fact that the S-protein, against which vaccine manufacturers are competing to develop a vaccine, shares a high degree of genetic and protein similarity (i.e. it is highly homologous in the sequence of nucleotides and amino acids) to two human proteins encoded by genes located on chromosomes 7 and 6, the so-called Sincitin-1 and Sincitin-2, respectively. (Figure 1)
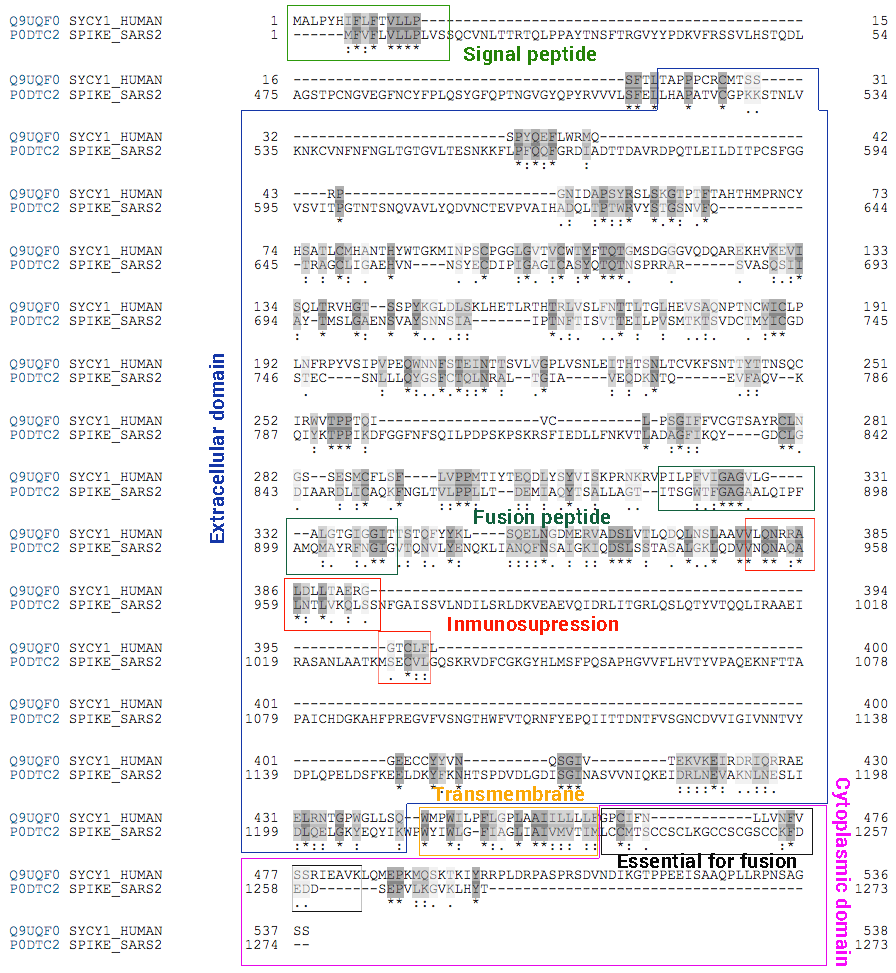
Sincitin-1 is the protein of the endogenous human retrovirus W (HERV-W) envelope, whose function is necessary during pregnancy to allow the development of the placenta and the differentiation of trophoblast 3, due to it intervenes in the fusion of the placental cells and allows the implantation of the embryo in the maternal uterus 4.
Sincitin-2 is the envelope protein of another member of the HERV family (HERV-FRD) and is also highly expressed in human placenta 5. Although both syncytins 1 and 2 are proteins that mediate the cell-cell fusion of cytrotrophs to allow the formation of the multinucleated layer of the syncytiotrophoblast during placental development, Sincitin- 2 (but not Sincitin- 1) has additional properties, an immunosuppressive activity that makes the foetus invisible to the mother’s immune system, thus preventing allogeneic rejection, since the embryo is a unique and unrepeatable human being, genetically different from the mother.
The similarity between the structure of the syncytins and the SARS-Cov-2 S-protein is truly striking. The protein of mature syncytins (envelope protein, Env, of endogenous human retroviruses, HERV) consists of a tritium of heterodimers of two subunits, S1 and S2, linked by a labile disulfide bond between the two chains, which is cleaved by Furine after S1 binding at receptor 7, 8, 9. (Figure 2)
The structure of the syncytins is the same as that described for the SARS-CoV-2 S protein. The S1 subunit of the spike binds to the receptor and then the separation between the two – the cut made by the enzyme Furine from the S1 and S2 subunits – allows the virus to enter the cells 10.
Interestingly, the SARS-CoV-2 virus also has sequences identical to
the syncytins that give it immunosuppressive activity 11, with which
the virus manages to make itself «invisible» to the immune system of the infected person.
Why could COVID-19 vaccines affect human fertility?
Firstly, experimental vaccines against COVID-19 could affect human fertility due to the high similarity between syncytins and the spike protein of SARS-CoV-2 11.
We do not yet know whether the antibodies generated by the action of the COVID-19 vaccination could cross react with the syncytins. If the antibodies against SARS-COV-2 recognise the human syncytins, these proteins would be blocked and neutralised by the antibodies, thus rendering them incapable of performing their function of fusing fetal cytotrophosphates, which play a key role in both the embryo implantation process and placental development. The result would be a miscarriage of the embryo in the vaccinated women, as the differentiation and nesting process in the mother’s womb is prevented by a direct inhibition of syncytins by antibodies induced by artificial immunisation with any of the experimental COVID-19 vaccines.
In fact, this statement is supported by the observation that the expression of recombinant syncytin in a wide variety of cell types induces the formation of giant syncites and the fusion of a human trophoblastic cell line expressing endogenous syncytin can be inhibited by an anti-synthetic antiserum. A rabbit polyclonal antibody produced against a mixture of Env-W peptics was able to inhibit in vitro cell fusion mediated by human syncytins 12.
With the same logic, we could expect that antibodies directed against the spike could also cross recognise and neutralise Sincitin 2, and thus its immunosuppressive activity could be affected, leaving the embryo exposed to the recognition of the mother’s immune system, which could lead to maternal immune rejection of the foetus 13.
It has been shown that the proteins of the HERV envelope (HERV-Env), on the one hand, trigger both innate and adaptive immunity, causing inflammatory, cytotoxic and apoptotic reactions. On the other hand, they have the capacity to prevent the activation of the immune response, presenting immunosuppressive properties and acting as immunoregulators 14.
When there is such a high similarity to endogenous retroviral peptide motifs, the human immune system can detect it as a distinct antigen and can trigger an allergic response, such as those that occur when haptens (e.g. penicillin) bind to host proteins 11.
With vaccination against COVID-19, IgE-type antibody responses and delayed-type hypersensitivity by T cells could also be induced, as was observed in mice, which after exposure to a SARS vaccine, caused them to have an allergic response 15, 16.
Therefore, we already know that due to the similarity of the spike protein of the SARS-CoV-2 virus with the two human proteins, Sincitin 1 and Sincitin 2, it is unlikely that a safe COVID-19 vaccine will be obtained, without observing allergic, cytotoxic and/or autoimmune side effects and without these effects affecting sooner or later the delicate mechanism of human reproduction.
Secondly, experimental vaccines against COVID-19 could affect human fertility because the levels of expression of messenger ribonucleic acid (mRNA) from syncytins increase progressively from the beginning of conception, during the first trimester and until the end of the pregnancy 17.
Both fusion and differentiation of trophoblast cells are associated with a concomitant increase in mRNA expression of the syncytin gene (HERV-W env) and the protein Sincitin. In simple terms, if the amount of protein or mRNA of the Syncytin gene decreases, defects in placental formation, poor trophoblast differentiation and vascular dysfunction in the placenta are observed.
Protein and transcription levels of the syncytin gene have been shown to be significantly decreased in placentas of women with pregnancy-induced hypertension, including patients with pre-eclampsia and gestational hypertension 18.
Clinically, diminished expression and abnormal localisation of Sincitin-1 and Sincitin-2 was found in pre-eclampsia, a disorder of pregnancy characterised by defects in placental formation, poor trophoblast differentiation and vascular dysfunction in the placenta. This means that altered expression of the syncytin gene and altered cell location of its protein product may contribute to the aetiology of pre-eclampsia 19. In other words, reduced placental expression of syncytin may contribute to altered cell fusion processes during placentagenesis and altered placental function in hypertensive disorders of pregnancy 20.
On the other
hand, determination of Syncytin-1 in human
sperm and its receptor ASCT-2 in the human oocyte most likely suggests a role
of Syncytin-1 in the fusion of sperm and oocyte during fertilization 21. The receptor
ASCT-2, but not Syncytin-1, is expressed in oocytes and the level of mRNA
increases with increasing maturity of the oocytes. However, how gamete
fusion is carried out by syncytins and their receptors is still unclear.
COVID-19 vaccines: A large-scale human transgenesis experiment
The mRNA vaccines against COVID-19 from the companies Moderna, Pfizer/BioNtech, and CureVac contain spike protein messenger RNA, which are administered coated with polyethylene glycol lipid nanoparticles in order to evade the body’s mechanisms and allow them to enter the cells.
This modified RNA therapy platform is totally new, it is an experimental form of inoculation of foreign genes into the human body that cannot be called «vaccination» since it does not involve administering attenuated or inactivated pathogens as simple antigens that stimulate immunity. It is the inoculation into the human body of injectable synthetic gene variants, so that they can penetrate into human cells and make them produce the spike (S) protein of the virus. This represents a true transgenesis experiment, never before performed in the history of mankind in order to confer immunity against human-transmitted infectious-contagious diseases.
Biotechnology companies are struggling to replicate the fact that mRNA vaccines do not have the ability to enter the nucleus to modify the DNA. They explain that the mRNA in the vaccine will only encode the spike glycoprotein (S) and merely transcribe it into the cell cytoplasm. It is noteworthy that experts and advisors from national and international health organisations are refraining from mentioning the epigenetic regulatory mechanism of mRNA. The ability to directly regulate gene expression is a mechanism widely recognised by molecular biology: gene silencing mediated by ribonucleic acids, the so-called inhibitory RNA (iRNA) 22.
The Nobel Assembly of the Karolinska Institutet in Stockholm, Sweden, awarded the 2006 Nobel Prize in Physiology or Medicine jointly to researchers Andrew Fire (Stanford University School of Medicine, Stanford, California, USA) and Craig Mello (University of Massachusetts Medical School, Worchester, Massachusetts, USA) for their discovery of RNA interference. These scientists demonstrated gene silencing through the use of double-stranded iRNA 23.
Through the mechanism of gene silencing mediated by iRNA, mRNA vaccines have the potential capacity to modify human DNA by inducing or silencing different genes in our genome. Interference RNA is a fundamental mechanism for controlling the flow of genetic information in cells.
In the specific case of syncytins, the use of RNA inhibitors (siRNA and shRNA), i.e. the use of antisense oligonucleotides specific to the syncytin gene, has already shown that the gene can be silenced and that inhibition of the expression of the Env-W protein leads to a reduction in fusion and differentiation of the human trophoblast 3.
Through in vivo experiments on animals, tests were carried out on loss of function in the uterus of sheep by injection of antisense oligonucleotides on day 8 of pregnancy. The injections of these oligonucleotides blocked the production of protein from the ERV gene envelope in the sheep’s trophoectoderm.
Specific gene treatment to silence the expression of the ERV envelope gene in sheep inhibited the differentiation of giant binucleated trophoblast cells and led to the loss of gestation on day 20 in all sheep receiving antisense oligonucleotides 24.
It should be noted that endogenous retroviruses (ERVs) are abundant in the genomes of vertebrates and play a fundamental role in mammalian reproduction, particularly in placental morphogenesis and implantation, which is why a similar result could be expected from specific gene inhibition of syncytins 1 and 2 in humans and primates 25 andpossibly of the two related env genes, syncytin A and syncytin B, in mice 26.
Antisense oligonucleotides are designed to modulate the transfer of information from the gene to the protein, interfering with the function of the mRNA or pre-RNA. To achieve effective modulation of gene expression by antisense oligonucleotides, modifications of oligonucleotides that do not promote RNase-H degradation of the target RNA are used. For example, they are designed to specifically inhibit mRNA expression of the HERV envelope gene, so that they inhibit splicing and/or translation of mRNA by a steric blocking mechanism, which is independent of RNase-H. In addition to the effects of these inhibitory oligonucleotides in the short term, long-term gene regulation can be achieved by intracellularly expressed antisense RNA administered by viral vectors 27.
Knowing the high homology that exists between syncytin and the spike protein of the SARS-CoV-2 virus, and knowing that the oligonucleotide sequences that silence the human syncytin gene have 100% homology with the sequences of the spike gene introduced in the vaccines, no one can guarantee that the mRNA injections contained in the vaccines will not end up affecting the expression of the endogenous human genes Syncytin-1 and Syncytin-2.
These safety aspects and adverse effects of the VID-19 vaccines on human fertility are not being assessed in the pre-clinical animal trials, nor in the phase I, II and III clinical trials already being carried out with volunteers who are not properly informed of all the risks involved in vaccination 28.
It is important to note that there are authors who point out that syncytin genes are present in humans and Old World primates and differ from Env genes which are present in rodents 17, 25. In this regard, pre-clinical trials of one of the mRNA candidate vaccines were conducted only in mice and hamsters and were published online at the end of October 2020 without peer review 29 after having started phase I and II clinical trials with volunteers.
Furthermore, the confidentiality clauses that have been granted by governments to the companies developing these vaccines will not allow us to know whether the constructs that make up the vectorised vaccines encode a SARS-CoV-2 spike gene and/or a single- or double-stranded iRNA with resistance to nucleases. Therefore, we also cannot know for sure if the vaccines that introduce modified RNAs have iRNA function and if they can target specific sites along the RNA transcription of a gene, in this case, the human syncytins, because of the high similarity they share in the sequence.
For all these reasons, the result of the inoculation of these experimental vaccines may end up leading to the production of antibodies «with 95% effectiveness» but it cannot be ruled out that, as a side effect, they may block the translation of a messenger RNA encoding a normal human protein. We know in advance that RNase H-resistant antisense oligonucleotides provide complete resistance to nucleases, exhibit good targeting ability, high efficiency in the cell and have sequence specificity 30.
With the molecular biology tools available today, biotechnology companies can introduce destabilising modifications to mRNAs, can improve the effectiveness of inhibitory RNAs and can thus activate an alternative mechanism through which the sensed strand is removed, giving iRNA powerful silencing activity. If these modified RNAs are administered with the VID-19 vaccines, the world’s population will be subjected to a new and overlooked method of experimental gene therapy on a large scale, aimed at destabilising the expression of human genes by injecting foreign sequences, with possible resistance to nucleases and a proven ability to exert epigenic control.
The companies developing these vaccines are not acting ethically or responsibly, because they are not conducting safety studies in the appropriate animal models, nor are they respecting the timescales needed to observe severe adverse effects in the medium and long term, nor are they providing the necessary information that they consider «confidential». By avoiding and improvising the pre-clinical testing phases and moving directly to the clinical testing phases I, II and III, companies are shifting the risk from animals to humans, using people as models of animal challenge.
In short, we are forced to denounce that if governments want to implement a massive and obligatory experimental vaccination of the population with vaccines that have not fulfilled the experimental phases and that are approved with «emergency» protocols, they are being complicit in possible crimes against humanity, because these ‘novel’ therapeutic platforms have the most widely accepted mechanisms of inhibitor RNA-induced gene silencing implicitly and invisibly in their designs, the effects of which are well known to the international scientific community and yet are being minimised by pharmaceutical companies, when they should be evaluated before these vaccines are commercially authorised.
The consequences of inoculating these foreign genes into the population with the VID-19 vaccines could be catastrophic for the fate of humanity, if we consider the role of HERV envelope proteins (syncytins) in human physiology and their possible pathogenic effects on several types of cancers and autoimmune disorders such as Multiple Sclerosis 31, 32, Amyotrophic Lateral Sclerosis 33, 34, 35 and Diabetes type 1 36.
Declaration of conflicts of interest
The author and the editor have no conflict of interest to declare.
References:
1- Wodarg W. https://www.wodarg.com/impfen/
2- Wodarg W and Yeadon M. Petition to European Medicines Agency Committee for human medicinal products (CHMP) COVID-19 EMA pandemic Task Force (COVID-ETF) Domenico Scarlattilaan 61083 HS Amsterdam The Netherlands. December 1, 2020. https://2020news.de/wp-content/uploads/2020/12/Wodarg_Yeadon_EMA_Petition_Pfizer_Trial_FINAL_01DEC2020_EN_unsigned_with_Exhibits.pdf
3- Frendo JL, Olivier D, Cheynet V, Blond JL, Bouton O, Vidaud M, Rabreau M, Evain-Brion D, Mallet F. Direct involvement of HERV-W Env glycoprotein in human trophoblast cell fusion and differentiation. Mol Cell Biol 2003; 23:3566–3574. PMID: 12724415 PMCID: PMC164757 DOI: 10.1128/mcb.23.10.3566-3574.2003 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC164757/pdf/1493.pdf
4- Mi, S., Lee, X., Li, Xp. et al. Syncytin is a captive retroviral envelope protein involved in human placental morphogenesis . Nature 403, 785–789 (2000). PMID: 10693809 DOI: 10.1038/35001608. https://www.nature.com/articles/35001608
5- Blaise S, de Parseval N, Bénit L, Heidmann T. Genomewide screening for fusogenic human endogenous retrovirus envelopes identifies syncytin 2, a gene conserved on primate evolution. Proc Natl Acad Sci U S A. 2003 Oct 28;100(22):13013-8. doi: 10.1073/pnas.2132646100. Epub 2003 Oct 13. PMID: 14557543; PMCID: PMC240736. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC240736/pdf/10013013.pdf
6- Mangeney M, Renard M, Schlecht-Louf G, Bouallaga I, Heidmann O, Letzelter C, Richaud A, Ducos B, Heidmann T. Placental syncytins: genetic disjunction between the fusogenic and immunosuppressive activity of retroviral envelope proteins. Proc Natl Acad Sci U S A 2007; 104:20534–20539. PMID: 18077339 PMCID: PMC2154466 DOI: 10.1073/pnas.0707873105. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2154466/pdf/zpq20534.pdf
7- Harris JR. Placental endogenous retrovirus (ERV): structural, functional, and evolutionary significance. Bioessays. 1998; 20: 307-316 PMID: 9619102 DOI: 10.1002/(SICI)1521-1878(199804)20:4<307::AID-BIES7>3.0.CO;2-M https://pubmed.ncbi.nlm.nih.gov/9619102/
8- Gong R, Peng X, Kang S, Feng H, Huang J, Zhang W, Lin D, Tien P, Xiao G. Structural characterization of the fusion core in syncytin, envelope protein of human endogenous retrovirus family W. Biochem Biophys Res Commun. 2005 Jun 17;331(4):1193-200. doi: 10.1016/j.bbrc.2005.04.032. PMID: 15883002; PMCID: PMC7092852. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7092852/pdf/main.pdf
9- Cheynet V, Ruggieri A, Oriol G, Blond JL, Boson B, Vachot L, Verrier B, Cosset FL, Mallet F. Synthesis, assembly, and processing of the Env ERVWE1/syncytin human endogenous retroviral envelope. J Virol. 2005 May;79(9):5585-93. doi: 10.1128/JVI.79.9.5585-5593.2005. PMID: 15827173; PMCID: PMC1082723. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1082723/pdf/1588-04.pdf
10- Walls AC, Park YJ, Tortorici MA, Wall A, McGuire AT and Veesler D. Structure, Function, and Antigenicity of the SARS-CoV-2 Spike Glycoprotein. Cell Volume 181, Issue 2, 16 April 2020, Pages 281-292. https://doi.org/10.1016/j.cell.2020.02.058
11- Gallaher B. Response to nCoV2019 Against Backdrop of Endogenous Retroviruses. https://virological.org/t/response-to-ncov2019-against-backdrop-of-endogenous-retroviruses/396
12- Mi S Xinhua L Xiang-Ping L Geertrudia MV Finnerty H Racie L et al. Syncytin is a captive retroviral envelope protein involved in human placental morphogenesis. Nature. 2000; 403: 785-789 PMID: 10693809 DOI: 10.1038/35001608 https://www.nature.com/articles/35001608
13- Chie-Pein Chen, Liang-Fu Chen, Su-Ray Yang, Chia-Yu Chen, Chun-Chuan Ko, Geen-Dong Chang, Hungwen Chen, Functional Characterization of the Human Placental Fusogenic Membrane Protein Syncytin 2, Biology of Reproduction, Volume 79, Issue 5, 1 November 2008, Pages 815–823, https://doi.org/10.1095/biolreprod.108.069765
14- Grandi N, Tramontano E. HERV Envelope Proteins: Physiological Role and Pathogenic Potential in Cancer and Autoimmunity. Front Microbiol. 2018 Mar 14;9:462. doi: 10.3389/fmicb.2018.00462. PMID: 29593697; PMCID: PMC5861771. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5861771/pdf/fmicb-09-00462.pdf
15- Tseng CT, Sbrana E, Iwata-Yoshikawa N, Newman PC, Garron T, Atmar RL, Peters CJ, Couch RB. Immunization with SARS coronavirus vaccines leads to pulmonary immunopathology on challenge with the SARS virus. PLoS One. 2012;7(4):e35421. doi: 10.1371/journal.pone.0035421. PMID: 22536382; PMCID: PMC3335060. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3335060/pdf/pone.0035421.pdf
16- Bolles M, Deming D, Long K, Agnihothram S, Whitmore A, Ferris M, Funkhouser W, Gralinski L, Totura A, Heise M, Baric RS. A double-inactivated severe acute respiratory syndrome coronavirus vaccine provides incomplete protection in mice and induces increased eosinophilic proinflammatory pulmonary response upon challenge. J Virol. 2011 Dec;85(23):12201-15. doi: 10.1128/JVI.06048-11. PMID: 21937658; PMCID: PMC3209347. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3209347/pdf/zjv12201.pdf
17- Keith JC Jr, Pijnenborg R, Van Assche FA. Placental syncytin expression in normal and preeclamptic pregnancies. Am J Obstet Gynecol. 2002 Oct;187(4):1122-3; author reply 1123-4. doi: 10.1067/mob.2002.128512. PMID: 12389018. https://www.ajog.org/article/S0002-9378(02)70072-0/fulltext
18- Knerr I, Beinder E, Rascher W. Syncytin, a novel human endogenous retroviral gene in human placenta: evidence for its dysregulation in preeclampsia and HELLP syndrome. Am J Obstet Gynecol 2002; 186:210–213. PMID: 11854637 DOI: 10.1067/mob.2002.119636 https://www.ajog.org/article/S0002-9378(02)11228-2/fulltext
19- Lee X, Keith JC Jr, Stumm N, Moutsatsos I, McCoy JM, Crum CP, Genest D, Chin D, Ehrenfels C, Pijnenborg R, van Assche FA, Mi S. Downregulation of placental syncytin expression and abnormal protein localization in pre-eclampsia. Placenta 2001; 22:808-812. PMID: 11718567 DOI: 10.1053/plac.2001.0722 https://linkinghub.elsevier.com/retrieve/pii/S0143-4004(01)90722-2
20- Chen CP, Wang KG, Chen CY, Yu C, Chuang HC, Chen H. Altered placental syncytin and its receptor ASCT2 expression in placental development and pre-eclampsia. BJOG 2006; 113:152–158. PMID: 16411991 DOI: 10.1111/j.1471-0528.2005.00843.x. https://obgyn.onlinelibrary.wiley.com/doi/full/10.1111/j.1471-0528.2005.00843.x
21- Bjerregaard, B., Lemmen, J.G., Petersen, M.R. et al. Syncytin-1 and its receptor is present in human gametes. J Assist Reprod Genet 31, 533–539 (2014). https://doi.org/10.1007/s10815-014-0224-1
22- Cavagnari BM. Regulación de la expresión génica: cómo operan los mecanismos epigenéticos. Regulation of gene expression: how do epigenetic mechanisms work. Arch Argent Pediatr 2012;110(2):132-136. Departamento de Pediatría. Hospital Alemán.Ciudad Autónoma de Buenos Aires :https://www.sap.org.ar/docs/publicaciones/archivosarg/2012/v110n2a08.pdf
23- The Nobel Prize in Physiology or Medicine 2006. NobelPrize.org. https://www.nobelprize.org/prizes/medicine/2006/7474-the-nobel-prize-in-physiology-or-medicine-2006-2006-4/
24- Dunlap KA, Palmarini M, Varela M, Burghardt RC, Hayashi K, Farmer JL, and Spencer TE. Endogenous retroviruses regulate periimplantation placental growth and differentiation PNAS September 26, 2006 103 (39) 14390-14395; https://doi.org/10.1073/pnas.0603836103 https://www.pnas.org/content/pnas/103/39/14390.full.pdf
25- Voisset C, Blancher A, Perron H, Mandrand B, Mallet F, Paranhos-Baccala G. Phylogeny of a novel family of human endogenous retrovirus sequences, HERV-W, in humans and other primates. AIDS Res Hum Retroviruses. 1999; 15: 1529-1533(15-19) PMID: 10580403 DOI: 10.1089/088922299309810 https://pubmed.ncbi.nlm.nih.gov/10580403/
26- Henke C, Ruebner M, Faschingbauer F, et al. Regulation of murine placentogenesis by the retroviral genes Syncytin-A, Syncytin-B and Peg10. Differentiation; Research in Biological Diversity. 2013 Apr-Jun;85(4-5):150-160. DOI: 10.1016/j.diff.2013.02.002. https://europepmc.org/article/med/23807393
27- Short-term and long-term modulation of gene expression by antisense therapeutics Peter Sazani, Marla M Vacek and Ryszard Kole. Current Opinion in Biotechnology Volume 13, Issue 5, 1 October 2002, Pages 468-472.https://doi.org/10.1016/S0958-1669(02)00366-X https://pubmed.ncbi.nlm.nih.gov/12459339/
28- Cardozo T and Veazey R . Informed consent disclosure to vaccine trial subjects of risk of COVID‐19 vaccines worsening clinical disease. International Journal of Clinical Practice, October 28, 2020 DOI: 10.111/ijcp.13795 https://doi.org/10.1111/ijcp.13795 https://onlinelibrary.wiley.com/doi/10.1111/ijcp.13795
29- Susanne Rauch, Nicole Roth, Kim Schwendt, et al. mRNA based SARS-CoV-2 vaccine candidate CVnCoV induces high levels of virus neutralizing antibodies and mediates protection in rodents. doi: https://doi.org/10.1101/2020.10.23.351775 https://www.biorxiv.org/content/10.1101/2020.10.23.351775v1.full.pdf
30- Summerton J. Morpholino antisense oligomers: the case for an RNase H-independent structural type. Biochim Biophys Acta. 1999 Dec 10;1489(1):141-58. doi: 10.1016/s0167-4781(99)00150-5. PMID: 10807004.https://pubmed.ncbi.nlm.nih.gov/10807004/
31- Dolei A. The aliens inside us: HERV-W endogenous retroviruses and multiple sclerosis. Mult Scler. 2018 Jan;24(1):42-47. doi: 10.1177/1352458517737370. PMID: 29307292. https://pubmed.ncbi.nlm.nih.gov/29307292/
32- Antony JM, Deslauriers AM, Bhat RK, Ellestad KK, Power C. Human endogenous retroviruses and multiple sclerosis: innocent bystanders or disease determinants? Biochim Biophys Acta. 2011 Feb;1812(2):162-76. doi: 10.1016/j.bbadis.2010.07.016. Epub 2010 Aug 6. PMID: 20696240; PMCID: PMC7172332. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7172332/pdf/main.pdf
33- Arru G, Mameli G, Deiana GA, Rassu AL, Piredda R, Sechi E, Caggiu E, Bo M, Nako E, Urso D, Mariotto S, Ferrari S, Zanusso G, Monaco S, Sechi G, Sechi LA. Humoral immunity response to human endogenous retroviruses K/W differentiates between amyotrophic lateral sclerosis and other neurological diseases. Eur J Neurol. 2018 Aug;25(8):1076-e84. doi: 10.1111/ene.13648. Epub 2018 May 14. PMID: 29603839. https://pubmed.ncbi.nlm.nih.gov/29603839/
34- Alfahad T, Nath A. Retroviruses and amyotrophic lateral sclerosis. Antiviral Res. 2013 Aug;99(2):180-7. doi: 10.1016/j.antiviral.2013.05.006. Epub 2013 May 23. PMID: 23707220; PMCID: PMC3723705. https://pubmed.ncbi.nlm.nih.gov/23707220/
35- Küry P, Nath A, Créange A, Dolei A, Marche P, Gold J, Giovannoni G, Hartung HP, Perron H. Human Endogenous Retroviruses in Neurological Diseases. Trends Mol Med. 2018 Apr;24(4):379-394. doi: 10.1016/j.molmed.2018.02.007. Epub 2018 Mar 15. PMID: 29551251; PMCID: PMC7185488. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7185488/pdf/main.pdf
36- Levet S, Charvet B, Bertin A, Deschaumes A, Perron H, Hober D. Human Endogenous Retroviruses and Type 1 Diabetes. Curr Diab Rep. 2019 Nov 21;19(12):141. doi: 10.1007/s11892-019-1256-9. PMID: 31754894; PMCID: PMC6872510. https://pubmed.ncbi.nlm.nih.gov/31754894/




