Varios grupos científicos han colaborado para proponer este proyecto de ley titulado «Ley de Seguridad y Soberanía de ADN/ADN». Su propósito es proteger la salud, la seguridad y la autonomía de las personas prohibiendo el uso, la venta y la distribución entre humanos, animales y productos agrícolas a base de ADN/ARN dentro del estado o provincia de su país, incluso durante emergencias declaradas.
Intención legislativa y justificación
Cada ciudadano desde su lugar puede aprovechar la información de este proyecto de ley para lograr que las autoridades reconozcan los riesgos significativos asociados con estas tecnologías, incluidos los efectos adversos para la salud, los datos de seguridad insuficientes a largo plazo y los posibles impactos ambientales, esta legislación garantiza protecciones sólidas, independientemente de las circunstancias que de otro modo podrían permitir accesos directos regulatorios o una supervisión disminuida durante emergencias de salud pública.
Al abordar estas preocupaciones y defender los principios de consentimiento informado, transparencia e integridad científica, esta Ley afirma el compromiso de [su pais] de salvaguardar a sus ciudadanos, animales y sistemas agrícolas de intervenciones farmacéuticas no probadas o potencialmente peligrosas.
Definición de inyecciones basadas en ADN/ARN
Para los fines de este proyecto de ley, las inyecciones basadas en ADN/ARN» se refieren a todos los productos que tienen la intención de funcionar utilizando, modificando o transferiendo material genético para inducir una respuesta inmune. Esto incluye inyecciones basadas en genes desarrollados con tecnología de:
- ácido ribonucleico mensajero (ARNm),
- tecnología modificada de ácido ribonucleico (modRNA),
- tecnología de ácido ribonucleico mensajero (SARN) o
- tecnología de ácido desoxirribonucleico (ADN).
Además, la definición abarca productos de terapia génica humana que median sus efectos mediante la transcripción o traducción del material genético transferido o al alterar específicamente las secuencias genéticas humanas. Estos productos incluyen ácidos nucleicos como plásmidos y ácido ribonucleico transcrito in vitro (ARN); microorganismos genéticamente modificados como virus, bacterias y hongos; Nucleasas específicas del sitio diseñadas utilizadas para la edición del genoma humano; y células humanas genéticamente modificadas genéticamente.
Al definir las inyecciones basadas en ADN/ARN de esta manera, el proyecto de ley garantiza que todas las formas de inmunización genética basada en material y enfoques de terapia génica, incluidas las inyecciones directas de material genético y el uso de portadores genéticamente modificados, caíga bajo su jurisdicción.
Cumplimiento y sanciones
Cualquier individuo, entidad, corporación, agencia gubernamental u organización que fabrique, distribuya, administre o facilite la venta de inyecciones basadas en ADN/ARN en violación de esta Ley estará sujeto a sanciones civiles y penales.
Se impondrá una multa de no menos de u$s 500.000 por violación para cada caso de producción, venta o administración no autorizadas de inyecciones basadas en ADN/ARN dentro del estado o provincia, con cada acto de distribución o administración a un producto individual, animal o agrícola separado que constituye una violación separada.
Además, cualquier persona o entidad que viole a sabiendas y deliberadamente esta Ley será culpable de un delito grave punible por una multa de hasta $ 750.000 y/o prisión por un término mínimo de 15 años.
Autoridad legal y mecanismos de aplicación
El Fiscal General de [su provincia o país] y cualquier agencia de aplicación de la ley debidamente designada tendrán la autoridad para investigar y enjuiciar violaciones, traer acciones civiles para ordenar los delitos continuos y buscar la restitución de las personas, empresas o entidades agrícolas afectadas.
Derecho de acción privado
Además, cualquier residente de [su provincia o país] que esté sujeto a la administración de inyecciones basadas en ADN/ARN en violación de esta Ley puede traer una causa privada de acción contra las partes responsables para buscar daños compensatorios y punitivos, incluidos los honorarios de los abogados y los costos judiciales.
Protecciones e incentivos a denunciantes
Los denunciantes que informan violaciones estarán protegidos de las represalias y pueden ser elegibles para una recompensa de hasta el 30% de las multas recopiladas si su información conduce a una acción exitosa de aplicación.
Marco legal para la implementación
Las siguientes disposiciones establecen el marco legal necesario para promulgar y hacer cumplir estas protecciones:
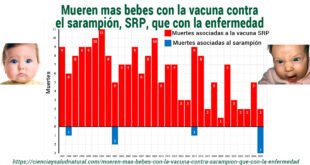
Mientras que, La evidencia sustancial revela que las inyecciones basadas en ADN/ARN son ineficaces para prevenir la infección, la transmisión, las enfermedades graves, la hospitalización o la muerte, con consecuencias adicionales sobre la inmunidady aumento de los riesgos asociados con su uso.
Las siguientes disposiciones establecen el marco jurídico necesario para promulgar y hacer cumplir estas protecciones.
- Efectividad
- Infección
- ‘Alarming Antibody Evasion Properties’—New COVID Subvariants Evade mRNA Vaccine-Induced Antibodies: Journal ‘Cell’
- Wang Q, Iketani S, Li Z, et al. Alarming antibody evasion properties of rising SARS-CoV-2 BQ and XBB subvariants. Cell. 2023;186(2):279-286.e8. doi:10.1016/j.cell.2022.12.018
- ‘Alarming Antibody Evasion Properties’—New COVID Subvariants Evade mRNA Vaccine-Induced Antibodies: Journal ‘Cell’
- Infección
- Pfizer COVID Jab Has ‘No Significant Effect’ Against Infection or Transmission: Journal ‘BMC Medicine’
- Althaus T, Overton CE, Devaux I, et al. How effective is the BNT162b2 mRNA vaccine against SARS-CoV-2 transmission and infection? A national programme analysis in Monaco, July 2021 to September 2022. BMC Med. 2024;22(1):227. Published 2024 Jun 5. doi:10.1186/s12916-024-03444-62 Doses of Pfizer COVID-19 Jab Only 3% Effective in Children, ‘Inadequate to Provide Protection Against’ New Variants: Journal ‘Immunology Letters’
- Rodrigues CO, Spinardi J, Rosa RG, et al. Real-world effectiveness of original BNT162b2 mRNA COVID-19 against symptomatic Omicron infection among children 5-11 years of age in Brazil: A prospective test-negative design study. Immunol Lett. 2024;269:106903. doi:10.1016/j.imlet.2024.106903
- Kirwan PD, Hall VJ, Foulkes S, et al. Effect of second booster vaccinations and prior infection against SARS-CoV-2 in the UK SIREN healthcare worker cohort. Lancet Reg Health Eur. 2023;36:100809. Published 2023 Dec 14. doi:10.1016/j.lanepe.2023.100809
- Lei J, MacNab Y. Bayesian hierarchical spatiotemporal models for prediction of (under)reporting rates and cases: COVID-19 infection among the older people in the United States during the 2020-2022 pandemic. Spat Spatiotemporal Epidemiol. 2024;49:100658. doi:10.1016/j.sste.2024.100658
- Ramya Sree A, Sethumadhavan K, Pullakanam SPT, Usharani P. Evaluation of CoVid-19 infection among vaccinated and unvaccinated individuals using biochemical markers. Bioinformation. 2024;20(3):223-228. Published 2024 Mar 31. doi:10.6026/973206300200223
- Tang Y, Boribong BP, Swank ZN, et al. COVID-19 mRNA vaccines induce robust levels of IgG but limited amounts of IgA within the oronasopharynx of young children. Preprint. medRxiv. 2024;2024.04.15.24305767. Published 2024 Apr 16. doi:10.1101/2024.04.15.24305767
- Conti MG, Piano Mortari E, Nenna R, et al. SARS-CoV-2-specific mucosal immune response in vaccinated versus infected children. Front Cell Infect Microbiol. 2024;14:1231697. Published 2024 Mar 27. doi:10.3389/fcimb.2024.1231697
- Igyártó BZ, Qin Z. The mRNA-LNP vaccines – the good, the bad and the ugly?. Front Immunol. 2024;15:1336906. Published 2024 Feb 8. doi:10.3389/fimmu.2024.1336906
- Chalupka A, Richter L, Chakeri A, et al. Effectiveness of a fourth SARS-CoV-2 vaccine dose in previously infected individuals from Austria. Eur J Clin Invest. 2024;54(3):e14136. doi:10.1111/eci.14136
- Xing Z, Jeyanathan M. A next-generation inhalable dry powder COVID vaccine. Nature. 2023;624(7992):532-534. doi:10.1038/d41586-023-03557-7
- Kirwan PD, Hall VJ, Foulkes S, et al. Effect of second booster vaccinations and prior infection against SARS-CoV-2 in the UK SIREN healthcare worker cohort. Lancet Reg Health Eur. 2023;36:100809. Published 2023 Dec 14. doi:10.1016/j.lanepe.2023.100809
- Yoshimura M, Sakamoto A, Ozuru R, et al. Insufficient anti-spike RBD IgA responses after triple vaccination with intramuscular mRNA BNT162b2 vaccine against SARS-CoV-2. Heliyon. 2023;10(1):e23595. Published 2023 Dec 13. doi:10.1016/j.heliyon.2023.e23595
- Katsube K, Nagai T, Watanabe T. Recurrent Skin Rash, Epigastralgia, and Arthralgia After SARS-CoV-2 mRNA Immunization and Breakthrough Infection. Gastroenterology. 2024;167(2):e5-e8. doi:10.1053/j.gastro.2023.12.024
- Althaus T, Overton CE, Devaux I, et al. How effective is the BNT162b2 mRNA vaccine against SARS-CoV-2 transmission and infection? A national programme analysis in Monaco, July 2021 to September 2022. BMC Med. 2024;22(1):227. Published 2024 Jun 5. doi:10.1186/s12916-024-03444-6
- Lataster R. Anti-science case study: COVID-19 vaccines’ effectiveness and safety exaggerated. Public Health Pract (Oxf). 2024;7:100517. Published 2024 May 23. doi:10.1016/j.puhip.2024.100517
- Feldstein LR, Ruffin J, Wiegand R, et al. Protection From COVID-19 Vaccination and Prior SARS-CoV-2 Infection Among Children Aged 6 Months-4 Years, United States, September 2022-April 2023. J Pediatric Infect Dis Soc. 2025;14(1):piae121. doi:10.1093/jpids/piae121
- Althaus T, Overton CE, Devaux I, et al. How effective is the BNT162b2 mRNA vaccine against SARS-CoV-2 transmission and infection? A national programme analysis in Monaco, July 2021 to September 2022. BMC Med. 2024;22(1):227. Published 2024 Jun 5. doi:10.1186/s12916-024-03444-62 Doses of Pfizer COVID-19 Jab Only 3% Effective in Children, ‘Inadequate to Provide Protection Against’ New Variants: Journal ‘Immunology Letters’
- Transmisión
- Breakthrough Infections in Healthcare Workers—1 in 110 After First Dose, 1 in 12 After Second, and 1 in 8 After Booster: Journal ‘Cureus’
- Chaudhary A, Bansal P, Satija M, et al. Breakthrough COVID-19 Infection Among the General Community, Frontline Workers, and Healthcare Workers During the Second and Third Wave in North India: A Longitudinal Study. Cureus. 2024;16(4):e58860. Published 2024 Apr 23. doi:10.7759/cureus.58860
- Althaus T, Overton CE, Devaux I, et al. How effective is the BNT162b2 mRNA vaccine against SARS-CoV-2 transmission and infection? A national programme analysis in Monaco, July 2021 to September 2022. BMC Med. 2024;22(1):227. Published 2024 Jun 5. doi:10.1186/s12916-024-03444-6
- Kampf G. Does the COVID-19 Vaccination Reduce the Risk to Transmit SARS-CoV-2 to Others?. Adv Exp Med Biol. 2024;1457:247-264. doi:10.1007/978-3-031-61939-7_14
- Igyártó BZ, Qin Z. The mRNA-LNP vaccines – the good, the bad and the ugly?. Front Immunol. 2024;15:1336906. Published 2024 Feb 8. doi:10.3389/fimmu.2024.1336906
- Donzelli A, Berrino F. Duration of SARS-CoV-2 Culturable Virus Shedding in Children by Vaccination Status. JAMA Pediatr. 2024;178(4):420-421. doi:10.1001/jamapediatrics.2023.6604
- Breakthrough Infections in Healthcare Workers—1 in 110 After First Dose, 1 in 12 After Second, and 1 in 8 After Booster: Journal ‘Cureus’
- Enfermedad
- COVID Shot Does Not Protect From Long COVID: Journal ‘Open Forum Infectious Diseases’Swift MD, Breeher LE, Dierkhising R, et al. Association of COVID-19 Vaccination With Risk of Medically Attended Postacute Sequelae of COVID-19 During the Ancestral, Alpha, Delta, and Omicron Variant Eras. Open Forum Infect Dis. 2024;11(9):ofae495. Published 2024 Aug 28. doi:10.1093/ofid/ofae495COVID and Flu Vaccines Do Not Reduce Severe Illness, Hospitalization, or Death: Study PreprintKirsch S, Marik P, Rogers C, Cosgrove K, Mead MN. A Novel Practical Approach for Directly Assessing COVID-19 Vaccine Efficacy against Hospitalization. Preprints. Preprint posted online August 6, 2024. doi:10.20944/preprints202408.0338.v1Hospitalization156% Increase in COVID Hospitalizations After ‘Mass COVID-19 Vaccination’ Campaign in Iran: ‘Journal of Global Health’Razimoghadam M, Daroudi R, Yaseri M. The effectiveness of COVID-19 vaccination in preventing hospitalisation and mortality: A nationwide cross-sectional study in Iran. J Glob Health. 2024;14:05026. Published 2024 Sep 27. doi:10.7189/jogh.14.05026Waning ImmunitySecond COVID-19 Booster Ineffective for Elderly: ‘Journal of Medical Virology’Carretero D, Giménez E, Albert E, et al. SARS-CoV-2-Spike T-cell response after receiving one or two Wuhan-Hu-1-based mRNA COVID-19 vaccine booster doses in elderly nursing home residents. J Med Virol. 2024;96(7):e29790. doi:10.1002/jmv.297904th Vaccine Booster Less than 2% Effective Against COVID After Just 4 Months, While ‘Recent Previous Infection Provided More Sustained Protection’: 9,560-Patient Study in ‘The Lancet’Kirwan PD, Hall VJ, Foulkes S, et al. Effect of second booster vaccinations and prior infection against SARS-CoV-2 in the UK SIREN healthcare worker cohort. Lancet Reg Health Eur. 2023;36:100809. Published 2023 Dec 14. doi:10.1016/j.lanepe.2023.100809COVID-19 Vaccine Immunity Wanes After Three Months: Journal ‘Genomics & Informatics’Lee B, Song H, Apio C, et al. An analysis of the waning effect of COVID-19 vaccinations. Genomics Inform. 2023;21(4):e50. doi:10.5808/gi.23088
- ‘17 Million’ COVID-19 Vaccine Deaths Worldwide: Physicists, Microbiologist for ‘CORRELATION Research in the Public Interest’
- Rancourt DG, Baudin M, Hickey J, Mercier J. COVID-19 vaccine-associated mortality in the Southern Hemisphere. CORRELATION Research in the Public Interest. Report. September 17, 2023. Available at: https://correlation-canada.org/covid-19-vaccine-associated-mortality-in-the-Southern-Hemisphere/.
- ‘Autopsy Findings in Cases of Fatal COVID-19 Vaccine-Induced Myocarditis’: Journal ‘ESC Heart Failure’
- Hulscher N, Hodkinson R, Makis W, McCullough PA. Autopsy findings in cases of fatal COVID-19 vaccine-induced myocarditis. ESC Heart Fail. Published online January 14, 2024. doi:10.1002/ehf2.14680
- 80% of Deceased COVID-Infected Patients Are Vaccinated—Only 20% Are Unvaccinated: Journal ‘Antimicrobial Stewardship & Healthcare Epidemiology’
- Helanne H, Forsblom E, Kainulainen K, Järvinen A, Kortela E. Incidence and outcome of hospital-acquired COVID-19 infections in secondary and tertiary care hospitals in the era of COVID-19 vaccinations. Antimicrob Steward Healthc Epidemiol. 2023;3(1):e216. Published 2023 Nov 30. doi:10.1017/ash.2023.489
- COVID-19 Jab Plays ‘Causal Role’ in Seizure Deaths: Journal ‘Academic Forensic Pathology’
- Prahlow JA. Deaths Related to New-Onset Seizures After Vaccination. Acad Forensic Pathol. Published online November 24, 2024. doi:10.1177/19253621241297029
- ~278,000 Americans May Have Died From the COVID-19 Vaccine by December 2021: Journal ‘Science, Public Health Policy and the Law’
- Skidmore M. COVID-19 illness and vaccination experiences in social circles affect COVID-19 vaccination decisions. Sci Public Health Policy Law. 2023;4:208-226
- ‘COVID-19 Vaccination Uptake in Europe Has Led to Increasing 2022 All-Cause Mortality’: Journal ‘Asian Pacific Journal of Health Sciences’
- Aarstad J, Kvitastein OA. Is there a link between the 2021 COVID-19 vaccination uptake in Europe and 2022 excess all-cause mortality? Asian Pac J Health Sci. 2023;10(1):25-31. doi:10.21276/apjhs.2023.10.1.6
- Risks of COVID Vaccines and Boosters Outweigh the Benefits in Children, Young Adults, and Older Adults With Low Occupational Risk or Previous Coronavirus Exposure: ‘ResearchGate’ Preprint
- Pantazatos SP, Seligmann H. COVID vaccination and age-stratified all-cause mortality risk. Preprint. October 2021. doi:10.13140/RG.2.2.28257.43366/1
- ‘Deaths Related to New-Onset Seizures After Vaccination’: Journal ‘Academic Forensic Pathology’
- Prahlow JA. Deaths Related to New-Onset Seizures After Vaccination. Acad Forensic Pathol. Published online November 24, 2024. doi:10.1177/19253621241297029
Considerando que hay evidencia sustancial que indica que las inyecciones basadas en ADN/ARN están asociadas con eventos adversos generalizados y graves, incluidos miles de lesiones documentadas, casos no reportados y aumentos significativos en el daño relacionado con productos farmacéuticos y exceso de mortalidad.
- Eventos adversos (EA)/Hospitalizaciones/Muertes
- FDA/Pfizer Safety Data Confirms 1,200+ Diseases Linked to COVID-19 Shot—Texas Judge Denies Agency’s Request for 75 Years to Release Data
- Pfizer. 5.3.6 Cumulative Analysis of Post-authorization Adverse Event Reports of PF-07302048 (BNT162B2) Received Through 28-Feb-2021. April 30, 2021.
- Lazarus R, Klompas M. Electronic Support for Public Health–Vaccine Adverse Event Reporting System (ESP:VAERS). Final Report. Agency for Healthcare Research and Quality (AHRQ); 2011. Grant ID: R18 HS 017045.
- Lee S, Lee K, Park J, et al. Global burden of vaccine-associated hepatobiliary and gastrointestinal adverse drug reactions, 1967-2023: A comprehensive analysis of the international pharmacovigilance database. J Med Virol. 2024;96(7):e29792. doi:10.1002/jmv.29792
- European Medicines Agency. Clinical trial results for trial 2020-005226-28. EU Clinical Trials Register. Published 23 Nov 2023. Accessed February 13, 2025. https://www.clinicaltrialsregister.eu/ctr-search/trial/2020-005226-28/results
- Fürst T, Šourek P, Krátká Z, Janošek J. Batch-dependent safety of COVID-19 vaccines in the Czech Republic and comparison with data from Denmark. Eur J Clin Invest. 2024;54(10):e14271. doi:10.1111/eci.14271
- Igyártó BZ, Qin Z. The mRNA-LNP vaccines – the good, the bad and the ugly?. Front Immunol. 2024;15:1336906. Published 2024 Feb 8. doi:10.3389/fimmu.2024.1336906
- Montes-Grajales D, Garcia-Serna R, Mestres J. Impact of the COVID-19 pandemic on the spontaneous reporting and signal detection of adverse drug events. Sci Rep. 2023;13(1):18817. Published 2023 Nov 1. doi:10.1038/s41598-023-46275-w
- Tsang RS, Agrawal U, Joy M, et al. Adverse events after first and second doses of COVID-19 vaccination in England: a national vaccine surveillance platform self-controlled case series study. J R Soc Med. 2024;117(4):134-148. doi:10.1177/01410768231205430
- Shin J, Shim SR, Lee J, Ryu HS, Kim JY. Otorhinolaryngologic complications after COVID-19 vaccination, vaccine adverse event reporting system (VAERS). Front Public Health. 2024;11:1338862. Published 2024 Jan 10. doi:10.3389/fpubh.2023.1338862
- Mostert S, Hoogland M, Huibers M, Kaspers G. Excess mortality across countries in the Western World since the COVID-19 pandemic: ‘Our World in Data’ estimates of January 2020 to December 2022. BMJ Public Health. 2024;2:e000282. doi:10.1136/bmjph-2023-000282
- COVID-19 Injection Doubles Risk of Post-COVID Death After 1 Year: Journal ‘Frontiers’
- Rodrigues NCP, Andrade MKN. Evaluation of post-COVID mortality risk in cases classified as severe acute respiratory syndrome in Brazil: a longitudinal study for medium and long term. Front Med (Lausanne). 2024;11:1495428. Published 2024 Dec 18. doi:10.3389/fmed.2024.1495428
- FDA/Pfizer Safety Data Confirms 1,200+ Diseases Linked to COVID-19 Shot—Texas Judge Denies Agency’s Request for 75 Years to Release Data
Considerando que numerosas organizaciones de salud, profesionales médicos y gobiernos internacionales han pedido una moratoria inmediata sobre las intervenciones farmacéuticas de ARNm, citando graves preocupaciones de salud, riesgos documentados y la urgente necesidad de investigaciones exhaustivas sobre su seguridad y eficacia.
- Piden una moratoria de la vacunación contra la COVID-19
- Southwest Idaho Health District Board Pulls COVID-19 Vaccines from ClinicsDozens of Countries Demand ‘Immediate Suspension of COVID-19 Modified mRNA Vaccines’: NORTH GroupMcCullough Foundation, & MIT, Research Triangle Park, Independent Researchers Call for ‘Global Moratorium on the modmRNA-LNP-Based Platform’: Journal ‘International Journal of Vaccine Theory, Practice, and Research’
- Mead MN, Seneff S, Rose J, Wolfinger R, Hulscher N, McCullough PA. COVID-19 Modified mRNA “Vaccines”: Lessons Learned from Clinical Trials, Mass Vaccination, and the Bio-Pharmaceutical Complex, Part 2. Int J Vaccine Theory Pract Res. 2024;3(2):1275-1286. doi:10.56098/w66wjg87
- Rogers C, Thorp JA, Cosgrove K, McCullough PA. COVID-19 Vaccines: A Risk Factor for Cerebral Thrombotic Syndromes. Int J Innov Res Med Sci. 2024;9(11):621-627. doi:10.23958/ijirms/vol09-i11/1982
- Oldfield PR, Gutschi LM, McCullough PA, Speicher DJ. Pfizer/BioNTech’s COVID-19 modRNA Vaccines: Dangerous Genetic Mechanism of Action Released Before Sufficient Preclinical Testing. J Am Phys Surg. 2024;29(4):118-126.
- ‘More Than 81,000 Physicians, Scientists, Researchers, and Concerned Citizens, 240 Elected Government Officials, 17 Professional Public Health and Physician Organizations, 2 State Republican Parties, 17 Republican Party County Committees, and 6 Scientific Studies from Across the World Have Called for the Market Withdrawal of COVID-19 Vaccines’: Journal ‘Science, Public Health Policy and The Law’
- Oldfield PR, Gutschi LM, McCullough PA, Speicher DJ. Pfizer/BioNTech’s COVID-19 modRNA Vaccines: Dangerous Genetic Mechanism of Action Released Before Sufficient Preclinical Testing. J Am Phys Surg. 2024;29(4):118-126.
- Southwest Idaho Health District Board Pulls COVID-19 Vaccines from ClinicsDozens of Countries Demand ‘Immediate Suspension of COVID-19 Modified mRNA Vaccines’: NORTH GroupMcCullough Foundation, & MIT, Research Triangle Park, Independent Researchers Call for ‘Global Moratorium on the modmRNA-LNP-Based Platform’: Journal ‘International Journal of Vaccine Theory, Practice, and Research’
Considerando que el Cirujano General de Florida, Joseph Ladapo, ha desaconsejado públicamente el uso de inyecciones de ARNm contra la COVID-19 para 23 millones de floridanos, citando riesgos significativos que incluyen miocarditis, enfermedad autoinmune, efectividad negativa, mayor riesgo de infección, proteína de pico persistente, posible integración de ADN y preocupaciones de contaminación que involucran secuencias SV40, que aumentan la urgencia de tomar medidas de precaución para proteger la salud pública.
- El director general de salud pública de Florida, Joseph Ladapo, desaconseja las inyecciones de ARNm contra la COVID-19.
- Florida Surgeon General Advises Against Using mRNA COVID-19 Jabs for 23 Million Floridians, Citing Risk of Myocarditis, POTS, Autoimmune Disease, Negative Effectiveness and Increased Infection Risk, Persisting Spike Protein in Body, DNA Integration, Other ‘Unknown Risk’Florida Surgeon General Calls for Halt in Use of COVID-19 mRNA Vaccines for 23 Million Floridians, Citing DNA Integration RiskTop FDA Heads Admit ‘Risks of Vaccination’ After Florida Surgeon General Call for Halt in Use of COVID-19 mRNA Jabs: Letter in ‘Journal of the American Medical Association’
- Marks P, Califf R. Is Vaccination Approaching a Dangerous Tipping Point?. JAMA. 2024;331(4):283-284. doi:10.1001/jama.2023.27685
- COVID-19 Vaccines May Pose ‘Unique and Heightened Risk’ of Contaminated DNA Fragment Integration into Human Body: Florida Surgeon General
- Florida Surgeon General Advises Against Using mRNA COVID-19 Jabs for 23 Million Floridians, Citing Risk of Myocarditis, POTS, Autoimmune Disease, Negative Effectiveness and Increased Infection Risk, Persisting Spike Protein in Body, DNA Integration, Other ‘Unknown Risk’Florida Surgeon General Calls for Halt in Use of COVID-19 mRNA Vaccines for 23 Million Floridians, Citing DNA Integration RiskTop FDA Heads Admit ‘Risks of Vaccination’ After Florida Surgeon General Call for Halt in Use of COVID-19 mRNA Jabs: Letter in ‘Journal of the American Medical Association’
Considerando que las inyecciones basadas en ADN/ARN carecen de estudios de seguridad aleatorizados, doble ciego y controlados con placebo a largo plazo, y los principales vacunólogos admiten que estas vacunas no han sido probadas adecuadamente en cuanto a seguridad, lo que aumenta aún más las preocupaciones sobre su uso generalizado.
- No han sido probadas adecuadamente: no hay estudios de seguridad aleatorizados, controlados con placebo, doble ciego y a largo plazo
- Top Vaccinologist Admits Vaccines Are Not Properly Tested for Safety: ‘The New England Journal of Medicine’
- Salmon DA, Orenstein WA, Plotkin SA, Chen RT. Funding Postauthorization Vaccine-Safety Science. N Engl J Med. 2024;391(2):102-105. doi:10.1056/NEJMp2402379
- Top Vaccinologist Admits Vaccines Are Not Properly Tested for Safety: ‘The New England Journal of Medicine’
Se ha descubierto que las inyecciones basadas en ADN/ARN contienen niveles peligrosos de contaminación del ADN (que superan los límites permitidos por la FDA en más de 500 veces), incluidas secuencias SV40 vinculadas al cáncer, sin evidencia de evaluaciones adecuadas del riesgo de integración del ADN realizadas, lo que plantea riesgos significativos y únicos para la salud humana.
- Contaminación
- 516 Times More DNA Contamination in COVID Jab Than FDA Limit—SV40 Cancer Sequence and Risk of Human Genome ‘Integration’: French Study
- Raoult D. Confirmation of the presence of vaccine DNA in the Pfizer anti-COVID-19 vaccine. HAL Preprint. 2024. Available at: https://hal.science/hal-04778576v1.
- 516 Times More DNA Contamination in COVID Jab Than FDA Limit—SV40 Cancer Sequence and Risk of Human Genome ‘Integration’: French Study
- Pfizer COVID Jab’s Dangerous DNA Impurities ‘Exceed the Permitted Limit Value’ by ‘More Than 500 Times’: Peer-Reviewed Journal ‘Methods and Protocols’
- König B, Kirchner JO. Methodological Considerations Regarding the Quantification of DNA Impurities in the COVID-19 mRNA Vaccine Comirnaty®. Methods Protoc. 2024;7(41). doi:10.3390/mps7030041.
- Multiple Reports have Find DNA Contamination in COVID-19 Jabs, Reported Across Multiple Manufacturers, Vaccine Platforms, and Geographic Regions, Far Exceeds Regulatory Thresholds Recommend by European Medicines Agency (EMA) and U.S. Food and Drug Administration (FDA): Journal ‘Science, Public Health Policy and The Law’
- Hulscher N, Bowden MT, McCullough PA. Calls for Market Removal of COVID-19 Vaccines Intensify as Risks Far Outweigh Theoretical Benefits. Sci Public Health Policy Law. 2025;6:2019-2025.
- ‘BioNTech RNA-Based COVID-19 Injections Contain Large Amounts Of Residual DNA Including An SV40 Promoter/Enhancer Sequence’: Journal ‘Science, Public, Health Policy and The Law
- Kämmerer U, Schulz V, Steger K. BioNTech RNA-Based COVID-19 Injections Contain Large Amounts of Residual DNA Including an SV40 Promoter/Enhancer Sequence. Sci Public Health Policy Law. 2024;5:2019-2024.
Considerando que la presencia de SV40, una secuencia vinculada al cáncer, en las inyecciones de ARNm contra la COVID-19 ha suscitado importantes preocupaciones de seguridad, con evidencia de contaminación que excede los límites de la FDA y advertencias del Cirujano General de Florida sobre su posible riesgo de integración en el genoma humano.
- SV40
- 516 Times More DNA Contamination in COVID Jab Than FDA Limit—SV40 Cancer Sequence and Risk of Human Genome ‘Integration’: French Study
- Raoult D. Confirmation of the presence of vaccine DNA in the Pfizer anti-COVID-19 vaccine. HAL Preprint. 2024. Available at: https://hal.science/hal-04778576v1.
- ‘BioNTech RNA-Based COVID-19 Injections Contain Large Amounts Of Residual DNA Including An SV40 Promoter/Enhancer Sequence’: Journal ‘Science, Public, Health Policy and The Law
- Kämmerer U, Schulz V, Steger K. BioNTech RNA-Based COVID-19 Injections Contain Large Amounts of Residual DNA Including an SV40 Promoter/Enhancer Sequence. Sci Public Health Policy Law. 2024;5:2019-2024.
- 516 Times More DNA Contamination in COVID Jab Than FDA Limit—SV40 Cancer Sequence and Risk of Human Genome ‘Integration’: French Study
Considerando que las inyecciones basadas en ADN/ARN pueden suponer riesgos significativos de integración genética y que la evidencia plantea serias preocupaciones sobre la posibilidad de que fragmentos de ADN contaminados y ARNm producto se integren en el genoma humano, como lo destacan datos y advertencias recientes del Cirujano General de Florida.
- Integración Genetica (DNA/RNA)
- ‘Latest Data Raise Serious Concerns About the Safety and Effectiveness’ of COVID Jabs: Journal ‘Frontiers in Immunology’
- Igyártó BZ, Qin Z. The mRNA-LNP vaccines – the good, the bad and the ugly?. Front Immunol. 2024;15:1336906. Published 2024 Feb 8. doi:10.3389/fimmu.2024.1336906
- ‘Intracellular Reverse Transcription of Pfizer BioNTech COVID-19 mRNA Vaccine BNT162b2 In Vitro in Human Liver Cell Line’: Journal ‘Current Issues in Molecular Biology’
- Aldén M, Olofsson Falla F, Yang D, et al. Intracellular Reverse Transcription of Pfizer BioNTech COVID-19 mRNA Vaccine BNT162b2 In Vitro in Human Liver Cell Line. Curr Issues Mol Biol. 2022;44(3):1115-1126. Published 2022 Feb 25. doi:10.3390/cimb44030073
- ‘Latest Data Raise Serious Concerns About the Safety and Effectiveness’ of COVID Jabs: Journal ‘Frontiers in Immunology’
Considerando que los ingredientes utilizados en las inyecciones basadas en ADN/ARN, incluidas las nanopartículas lipídicas, la pseudouridina, SM-102, ALC-0315, PEG y trometamina, se han relacionado con toxicidad, carcinogenicidad, respuestas inmunitarias no deseadas y otros efectos nocivos, con preocupaciones adicionales con respecto a la persistencia de la proteína de pico patógena y la presencia de elementos químicos tóxicos no declarados..
- Toxicidad/carcinogenicidad de los ingredientes
- Lipid Nanotechnology
- Moderna Scientists Admit ‘Risks of mRNA Drug and Vaccine Toxicity’: Journal ‘Nature’
- Bitounis D, Jacquinet E, Rogers MA, Amiji MM. Strategies to reduce the risks of mRNA drug and vaccine toxicity. Nat Rev Drug Discov. 2024;23(4):281-300. doi:10.1038/s41573-023-00859-3
- Moderna Scientists Admit ‘Risks of mRNA Drug and Vaccine Toxicity’: Journal ‘Nature’
- Lipid Nanotechnology
- mRNA COVID Jab Ingredient N1-Methyl-Pseudouridine (m1Ψ) ‘Stimulated Cancer Growth and Metastasis’: ‘International Journal of Biological Macromolecules’
- Rubio-Casillas A, Cowley D, Raszek M, Uversky VN, Redwan EM. Review: N1-methyl-pseudouridine (m1Ψ): Friend or foe of cancer? [published correction appears in Int J Biol Macromol. 2024 Jun;270(Pt 2):132447. doi: 10.1016/j.ijbiomac.2024.132447.]. Int J Biol Macromol. 2024;267(Pt 1):131427. doi:10.1016/j.ijbiomac.2024.131427
- Roughly 1/3rd of mRNA COVID-19 Vaccine Recipients Experience ‘Unintended Immune Response in the Body’ Caused by ‘Frameshifting’: Cambridge University, Journal ‘Nature’
- Mulroney TE, Pöyry T, Yam-Puc JC, et al. N1-methylpseudouridylation of mRNA causes +1 ribosomal frameshifting. Nature. 2024;625(7993):189-194. doi:10.1038/s41586-023-06800-3
- Tromethamine
- PEG
- 100% of Animals Exposed to COVID Jab Ingredient ‘PEG’ Suffer Anaphylactic Shock Just 2 Minutes After Injection: Study in Journal ‘Vaccine: X’
- Barta BA, Radovits T, Dobos AB, et al. Comirnaty-induced cardiopulmonary distress and other symptoms of complement-mediated pseudo-anaphylaxis in a hyperimmune pig model: Causal role of anti-PEG antibodies. Vaccine X. 2024;19:100497. Published 2024 May 23. doi:10.1016/j.jvacx.2024.100497
- 100% of Animals Exposed to COVID Jab Ingredient ‘PEG’ Suffer Anaphylactic Shock Just 2 Minutes After Injection: Study in Journal ‘Vaccine: X’
- ‘COVID-19 Spike Protein Is Pathogenic, from Both Virus and Vaccine mRNA’: Journal ‘Biomedicines’
- Parry PI, Lefringhausen A, Turni C, et al. ‘Spikeopathy’: COVID-19 Spike Protein Is Pathogenic, from Both Virus and Vaccine mRNA. Biomedicines. 2023;11(8):2287. Published 2023 Aug 17. doi:10.3390/biomedicines11082287
- Spike Protein ‘Possible Primary Contributing Factor to Long COVID’: Journal ‘Cureus’
- Hulscher N, Procter BC, Wynn C, McCullough PA. Clinical Approach to Post-acute Sequelae After COVID-19 Infection and Vaccination. Cureus. 2023;15(11):e49204. Published 2023 Nov 21. doi:10.7759/cureus.49204
- Vaccine Spike Protein Found COVID-19 Vaccinated Thalamus, Pons, and Pituitary and Adrenal Glands: Journal ‘Pathology International’
- Mikami S, Ishii M, Yano T, et al. Multifocal meningoencephalitis after vaccination against COVID-19. Pathol Int. 2024;74(12):697-703. doi:10.1111/pin.13491
- Spike Protein in Vaccinated Deceased Heart Tissue—’Response to Vaccination’: ‘International Journal of Molecular Sciences’
- Baumeier C, Aleshcheva G, Harms D, et al. Intramyocardial Inflammation after COVID-19 Vaccination: An Endomyocardial Biopsy-Proven Case Series. Int J Mol Sci. 2022;23(13):6940. Published 2022 Jun 22. doi:10.3390/ijms23136940
- Vaccine-Derived Spike Protein Found in Blood Up to 6 Months Post-Vaccination: Journal ‘Proteomics Clinical Applications’
- Brogna C, Cristoni S, Marino G, et al. Detection of recombinant Spike protein in the blood of individuals vaccinated against SARS-CoV-2: Possible molecular mechanisms. Proteomics Clin Appl. 2023;17(6):e2300048. doi:10.1002/prca.202300048
Considerando que aproximadamente un tercio de los receptores de la inyecciones de ARNm contra la COVID-19 experimentan respuestas inmunes no deseadas causadas por un fenómeno conocido como «cambio de marco de lectura», como lo documentó la Universidad de Cambridge, lo que genera preocupaciones importantes sobre la seguridad y la previsibilidad de estas intervenciones.
- Frameshifting, Cambio de Marco
- Roughly 1/3rd of mRNA COVID-19 Vaccine Recipients Experience ‘Unintended Immune Response in the Body’ Caused by ‘Frameshifting’: Cambridge University, Journal ‘Nature’
- Mulroney TE, Pöyry T, Yam-Puc JC, et al. N1-methylpseudouridylation of mRNA causes +1 ribosomal frameshifting. Nature. 2024;625(7993):189-194. doi:10.1038/s41586-023-06800-3
- Roughly 1/3rd of mRNA COVID-19 Vaccine Recipients Experience ‘Unintended Immune Response in the Body’ Caused by ‘Frameshifting’: Cambridge University, Journal ‘Nature’
Considerando que las inyecciones basadas en ADN/ARN exhiben una biodistribución y bioacumulación de área amplia, con proteínas pico o spike y secuencias de ARNm detectadas en órganos críticos como el cerebro, el corazón y las glándulas endocrinas, así como en la sangre, la placenta y el feto, lo que genera serias preocupaciones sobre los efectos sistémicos y a largo plazo en la salud humana.
- Biodistribución/bioacumulación en áreas extensas
- Vaccine Spike Protein Found COVID-19 Vaccinated Thalamus, Pons, and Pituitary and Adrenal Glands: Journal ‘Pathology International’
- Mikami S, Ishii M, Yano T, et al. Multifocal meningoencephalitis after vaccination against COVID-19. Pathol Int. 2024;74(12):697-703. doi:10.1111/pin.13491
- Mörz M. A Case Report: Multifocal Necrotizing Encephalitis and Myocarditis after BNT162b2 mRNA Vaccination against COVID-19. Vaccines (Basel). 2022;10(10):1651. Published 2022 Oct 1. doi:10.3390/vaccines10101651
- Brogna C, Cristoni S, Marino G, et al. Detection of recombinant Spike protein in the blood of individuals vaccinated against SARS-CoV-2: Possible molecular mechanisms. Proteomics Clin Appl. 2023;17(6):e2300048. doi:10.1002/prca.202300048
- Hulscher N, McCullough PA, Marotta DE. Strategic deactivation of mRNA COVID-19 vaccines: New applications for siRNA therapy and RIBOTACs. J Gene Med. 2024;26(9):e3733. doi:10.1002/jgm.3733
- Zhong C, Cohen K, Lin X, Schiller E, Sharma S, Hanna N. COVID-19 Vaccine mRNA Biodistribution: Maternal and Fetal Exposure Risks. Am J Reprod Immunol. 2024;92(4):e13934. doi:10.1111/aji.13934
- Castruita JAS, Schneider UV, Mollerup S, et al. SARS-CoV-2 spike mRNA vaccine sequences circulate in blood up to 28 days after COVID-19 vaccination. APMIS. 2023;131(3):128-132. doi:10.1111/apm.13294
- Kent SJ, Li S, Amarasena TH, et al. Blood Distribution of SARS-CoV-2 Lipid Nanoparticle mRNA Vaccine in Humans. ACS Nano. 2024;18(39):27077-27089. doi:10.1021/acsnano.4c11652
- Vaccine Spike Protein Found COVID-19 Vaccinated Thalamus, Pons, and Pituitary and Adrenal Glands: Journal ‘Pathology International’
Considerando que se ha demostrado que los componentes de las inyecciones basadas en ADN/ARN, incluidas las proteínas de pico y las secuencias de ARNm, persisten en el cuerpo mucho más tiempo del que se afirmó inicialmente (hasta 245 días en algunos casos), lo que plantea preocupaciones críticas sobre sus efectos a largo plazo en la salud humana y la estabilidad sistémica.
- Persistencia de los componentes de la inyección
- COVID-19 Jab mRNA and Spike Protein Stay in Human Tissue Longer Than Expected—187 Days: Journal ‘British Pharmacological Society’
- Boros LG, Kyriakopoulos AM, Brogna C, Piscopo M, McCullough PA, Seneff S. Long-lasting, biochemically modified mRNA, and its frameshifted recombinant spike proteins in human tissues and circulation after COVID-19 vaccination. Pharmacol Res Perspect. 2024;12(3):e1218. doi:10.1002/prp2.1218
- Patterson BK, Yogendra R, Francisco EB, Long E, Pise A, Osgood E, Bream J, Kreimer M, Jeffers D, Beaty C, Vander Heide R, Guevara-Coto J, Mora-Rodríguez RA. Persistence of S1 Spike Protein in CD16+ Monocytes up to 245 Days in SARS-CoV-2 Negative Post COVID-19 Vaccination Individuals with Post-Acute Sequelae of COVID-19 (PASC)-Like Symptoms. medRxiv. Published March 24, 2024. doi:10.1101/2024.03.24.24304286.
- Castruita JAS, Schneider UV, Mollerup S, et al. SARS-CoV-2 spike mRNA vaccine sequences circulate in blood up to 28 days after COVID-19 vaccination. APMIS. 2023;131(3):128-132. doi:10.1111/apm.13294
- Kent SJ, Li S, Amarasena TH, et al. Blood Distribution of SARS-CoV-2 Lipid Nanoparticle mRNA Vaccine in Humans. ACS Nano. 2024;18(39):27077-27089. doi:10.1021/acsnano.4c11652
- Krauson AJ, Casimero FVC, Siddiquee Z, Stone JR. Duration of SARS-CoV-2 mRNA vaccine persistence and factors associated with cardiac involvement in recently vaccinated patients. NPJ Vaccines. 2023;8(1):141. Published 2023 Sep 27. doi:10.1038/s41541-023-00742-7
- COVID-19 Jab mRNA and Spike Protein Stay in Human Tissue Longer Than Expected—187 Days: Journal ‘British Pharmacological Society’
Las inyecciones basadas en ADN/ARN se han asociado fuertemente con eventos cardiovasculares y trombóticos graves, que incluyen miocarditis, pericarditis, coágulos sanguíneos y miocardiopatía, con aumentos documentados en los informes de eventos adversos y muertes súbitas, particularmente entre individuos jóvenes, lo que genera preocupaciones urgentes sobre su seguridad y uso generalizado.
- Eventos cardíacos/cardiovasculares/trombóticos: miocarditis/pericarditis/coágulos de sangre
- Worldwide Study of 130 Million Injuries from 1969 to 2023 Confirms ‘Increase in Pericarditis and Myocarditis Reports Associated With Vaccines’: WHO Data
- Lee S, Jo H, Lee H, et al. Global estimates on the reports of vaccine-associated myocarditis and pericarditis from 1969 to 2023: Findings with critical reanalysis from the WHO pharmacovigilance database. J Med Virol. 2024;96(6):e29693. doi:10.1002/jmv.29693
- Heart Disease Occurs ‘Only in the Vaccinated Groups’ in Children: Oxford/Harvard COVID Jab Study
- Andrews CD, Parker EP, Horne E, et al. OpenSAFELY: Effectiveness of COVID-19 vaccination in children and adolescents. medRxiv. Published May 20, 2024. doi:10.1101/2024.05.20.24306810.
- Cardiac Arrest Deaths Spike 1,236% from 2020 to 2023 in U.S. County Following COVID-19 Jab Campaign: Study
- Hulscher N, Cook MJ, Stricker RB, McCullough PA. Excess Cardiopulmonary Arrest and Mortality After COVID-19 Vaccination in King County, Washington. J Emerg Med OA. 2024;2(1):1-11.
- Man Suffers Skin Cancer, Heart Disease Following COVID-19 Vaccinations: ‘Frontiers in Oncology’ Case Report
- Nishizawa A, Kawakami M, Kitahara Y. Case report: A case of metastatic BRAFV600-mutated melanoma with heart failure treated with immune checkpoint inhibitors and BRAF/MEK inhibitors. Front Oncol. 2024;14:1366532. Published 2024 Mar 11. doi:10.3389/fonc.2024.1366532
- Myocarditis Risk 9x Greater Following 3rd COVID-19 Jab: ‘Journal of Evidence-Based Medicine’
- Lai D, Lim D, Lu J, Wang H, Huang T, Zhang YD. Risk of myocarditis after three doses of COVID-19 mRNA vaccines in the United States, 2020-2022: A self-controlled case series study. J Evid Based Med. 2024;17(1):65-77. doi:10.1111/jebm.12595
- Study of 99 Million COVID-Vaccinated Confirms Increased Risk for Cardiovascular and Neurological Disorders: Journal ‘Vaccine’
- Faksova K, Walsh D, Jiang Y, et al. COVID-19 vaccines and adverse events of special interest: A multinational Global Vaccine Data Network (GVDN) cohort study of 99 million vaccinated individuals. Vaccine. 2024;42(9):2200-2211. doi:10.1016/j.vaccine.2024.01.100
- Woman Dies After Spinal Cord Injury, Heart Attack Following COVID-19 Vaccination: Acute Necrotizing Myelitis (‘Annals of Medicine & Surgery’ Case Study)
- Rezvani M, Mahmoodkhani M, Sourani A, et al. Treatment refractory acute necrotizing myelitis after COVID-19 vaccine injection: a case report. Ann Med Surg (Lond). 2024;86(2):1185-1190. Published 2024 Jan 3. doi:10.1097/MS9.0000000000001662
- Blood Pooling Outside of Spinal Vessels (Hematoma) Following COVID-19 Vaccination: Journal ‘Annals of Medicine & Surgery’ Case Report
- Rezvani M, Sabouri M, Aminmansour B, et al. Spontaneous spinal epidural haematoma following COVID-19 vaccination: a case report. Ann Med Surg (Lond). 2023;86(1):612-619. Published 2023 Dec 8. doi:10.1097/MS9.0000000000001604
- ‘Autopsy Findings in Cases of Fatal COVID-19 Vaccine-Induced Myocarditis’: Journal ‘ESC Heart Failure’
- Hulscher N, Hodkinson R, Makis W, McCullough PA. Autopsy findings in cases of fatal COVID-19 vaccine-induced myocarditis. ESC Heart Fail. Published online January 14, 2024. doi:10.1002/ehf2.14680
- Hundreds of Serious Heart Rhythm Disturbances Following COVID Vaccination in U.S.: Journal ‘Heart Rhythm 02’
- Kumar A, Shariff M, Lee J, et al. Ventricular fibrillation and ventricular tachycardia post-SARS-CoV-2-targeted mRNA/viral vector vaccination. Heart Rhythm O2. 2023;4(12):826-828. Published 2023 Nov 2. doi:10.1016/j.hroo.2023.11.001
- COVID-19 Vaccine Induces ‘Broken Heart Syndrome’ (Cardiomyopathy): Journal ‘Cureus’
- Singh B, Manita B, Suman F, et al. A Systematic Review of COVID-19 Vaccine-Induced Takotsubo Cardiomyopathy: A 2023 Update. Cureus. 2023;15(12):e50319. Published 2023 Dec 11. doi:10.7759/cureus.50319
- Youth ER Visits, Hospitalizations for ‘Chest Pain’ Double After Vaccination: Peer-Reviewed Publication in ‘Translational Pediatrics’
- Tan JZ, Zhang DZ, Sundararaghavan S, et al. Chest pain attendances to a Paediatric Emergency Department pre- and post-COVID-19 vaccination. Transl Pediatr. 2023;12(11):2010-2019. doi:10.21037/tp-23-230
- Serious Cardiovascular, Respiratory, Immune, Digestive, Nervous System Conditions ‘Are Associated with Primary Vaccination Using COVID-19 mRNA Vaccines’: Peer-Reviewed Journal ‘Vaccine: X’
- Dorajoo SR, Tan HX, Teo CHD, et al. Nationwide safety surveillance of COVID-19 mRNA vaccines following primary series and first booster vaccination in Singapore. Vaccine X. 2023;15:100419. Published 2023 Dec 2. doi:10.1016/j.jvacx.2023.100419
- ‘Fatal Myocarditis following COVID-19 mRNA Immunization’: Journal ‘Vaccines’
- Sousa PMB, Silva EA, Campos MAG, Lages JS, Corrêa RDGCF, Silva GEB. Fatal Myocarditis following COVID-19 mRNA Immunization: A Case Report and Differential Diagnosis Review. Vaccines (Basel). 2024;12(2):194. Published 2024 Feb 13. doi:10.3390/vaccines12020194
- ‘Intramyocardial Inflammation after COVID-19 Vaccination’: ‘International Journal of Molecular Sciences’
- Baumeier C, Aleshcheva G, Harms D, et al. Intramyocardial Inflammation after COVID-19 Vaccination: An Endomyocardial Biopsy-Proven Case Series. Int J Mol Sci. 2022;23(13):6940. Published 2022 Jun 22. doi:10.3390/ijms23136940
- ‘SARS-CoV-2 Spike mRNA Vaccine Sequences Circulate in Blood Up to 28 Days After COVID-19 Vaccination’: Journal ‘AMPIS’
- Castruita JAS, Schneider UV, Mollerup S, et al. SARS-CoV-2 spike mRNA vaccine sequences circulate in blood up to 28 days after COVID-19 vaccination. APMIS. 2023;131(3):128-132. doi:10.1111/apm.13294
- ‘SARS-CoV-2 Spike mRNA Vaccine Sequences Were Identified in Plasma Up to 28 Days Postvaccination’: Journal ‘ACS Nano’
- Kent SJ, Li S, Amarasena TH, et al. Blood Distribution of SARS-CoV-2 Lipid Nanoparticle mRNA Vaccine in Humans. ACS Nano. 2024;18(39):27077-27089. doi:10.1021/acsnano.4c11652
- ‘SARS-CoV-2 mRNA Vaccines Routinely Persist Up to 30 Days from Vaccination and Can Be Detected in the Heart’: Nature’s ‘NPJ Vaccines’
- Krauson AJ, Casimero FVC, Siddiquee Z, Stone JR. Duration of SARS-CoV-2 mRNA vaccine persistence and factors associated with cardiac involvement in recently vaccinated patients. NPJ Vaccines. 2023;8(1):141. Published 2023 Sep 27. doi:10.1038/s41541-023-00742-7
- SARS-CoV2 mRNA Vaccine Intravenous Administration Induces Myocarditis in Chronic Inflammation: Journal ‘PLOS ONE’
- Jeon HE, Lee S, Lee J, et al. SARS-CoV2 mRNA vaccine intravenous administration induces myocarditis in chronic inflammation. PLoS One. 2024;19(10):e0311726. Published 2024 Oct 10. doi:10.1371/journal.pone.0311726
- Pfizer, Moderna COVID-19 Shots ‘Induce Specific Dysfunctions That Correlate Pathophysiologically to Cardiomyopathy’: Journal ‘British Pharmacological Society’
- Schreckenberg R, Woitasky N, Itani N, Czech L, Ferdinandy P, Schulz R. Cardiac side effects of RNA-based SARS-CoV-2 vaccines: Hidden cardiotoxic effects of mRNA-1273 and BNT162b2 on ventricular myocyte function and structure. Br J Pharmacol. 2024;181(3):345-361. doi:10.1111/bph.16262
- ‘COVID-19 Vaccines: A Risk Factor for Cerebral Thrombotic Syndromes’: ‘International Journal of Innovative Research in Medical Science’
- Rogers C, Thorp JA, Cosgrove K, McCullough PA. COVID-19 Vaccines: A Risk Factor for Cerebral Thrombotic Syndromes. Int J Innov Res Med Sci. 2024;9(11). doi:10.23958/ijirms/vol09-i11/1982.
- ‘Vaccine-Induced Immune Thrombotic Thrombocytopenia Post COVID-19 Booster Vaccination’—Study Advises Against Vaccination in Those <50 Years Who Haven’t Received a Previous Dose: Journal ‘Research and Practice in Thrombosis and Hemostasis’
- Mendes-de-Almeida DP; Brazilian VITT Investigative Collaboration Group, Mouta Nunes de Oliveira P, et al. Vaccine-induced immune thrombotic thrombocytopenia post COVID-19 booster vaccination in Brazil: a case series. Res Pract Thromb Haemost. 2023;7(8):102243. Published 2023 Oct 31. doi:10.1016/j.rpth.2023.102243
- Superior Mesenteric Vein Thrombosis Due to COVID-19 Vaccination: ‘Journal of Medical Case Reports’
- Suto K, Saito A, Mori K, Yoshida A, Sata N. Superior mesenteric vein thrombosis due to COVID-19 vaccination: a case report. J Med Case Rep. 2024;18(1):23. Published 2024 Jan 11. doi:10.1186/s13256-023-04320-2
- Acute Myocarditis Following COVID-19 mRNA Vaccination: Journal ‘Radiology Case Reports’
- Yeo SZJ, Ho CL. COVID-19 mRNA vaccine-related myocarditis: A PRISMA systematic review, imaging approach and differential diagnoses. Radiol Case Rep. 2023;19(3):1008-1019. Published 2023 Dec 22. doi:10.1016/j.radcr.2023.11.070
- CDC VAERS: Myocarditis Reports in 2021 After COVID-19 Vaccination Were 223x Higher Than 30-Year Vaccine Average, Marking a 2,500% Increase: Journal ‘Therapeutic Advances in Drug Safety’
- Rose J, Hulscher N, McCullough PA. Determinants of COVID-19 vaccine-induced myocarditis. Ther Adv Drug Saf. 2024;15:20420986241226566. Published 2024 Jan 27. doi:10.1177/20420986241226566
- ‘COVID-19 Vaccines-Associated Takotsubo Cardiomyopathy’: Journal ‘Le Infezioni in Medicina’
- Hassanzadeh S, Suleiman A, Correia JJ, Montazerin SM. COVID-19 vaccines-associated Takotsubo cardiomyopathy: A narrative review. Infez Med. 2024;32(1):1-11. Published 2024 Mar 1. doi:10.53854/liim-3201-1
- Myocarditis Is a Safety Concern Following COVID-19 mRNA Vaccination: ‘Journal of Evidence-Based Medicine’
- Lai D, Lim D, Lu J, Wang H, Huang T, Zhang YD. Risk of myocarditis after three doses of COVID-19 mRNA vaccines in the United States, 2020-2022: A self-controlled case series study. J Evid Based Med. 2024;17(1):65-77. doi:10.1111/jebm.12595
- Myocarditis Confirmed as ‘Possible Complication of Vaccination with a SARS-CoV2 mRNA Vaccine: Journal ‘Clinical Medicine’
- Sularz AK, Hua A, Ismail T. SARS-CoV-2 vaccines and myocarditis. Clin Med (Lond). 2023;23(5):495-502. doi:10.7861/clinmed.2023-0049
- ‘Autopsy Case of Reversible Cerebral Vasoconstriction Syndrome After a Severe Acute Respiratory Syndrome Coronavirus 2 Vaccination’: Journal ‘Cureus’
- Shimura M, Fujikawa H, Yazawa M, Matsumoto Y, Yamada M. An Autopsy Case of Reversible Cerebral Vasoconstriction Syndrome After a Severe Acute Respiratory Syndrome Coronavirus 2 Vaccination. Cureus. 2024;16(4):e59311. Published 2024 Apr 29. doi:10.7759/cureus.59311
- ‘Transient Aortitis after COVID-19 mRNA Vaccination’: ‘Mediterranean Journal of Rheumatology’
- Nishioka H, Okumura Y. Transient Aortitis after COVID-19 mRNA Vaccination. Mediterr J Rheumatol. 2023;35(1):195-196. Published 2023 Oct 20. doi:10.31138/mjr.231020.nht
- ‘COVID-19 mRNA Vaccination-Induced Myopericarditis in an Otherwise Healthy Young Male’: Journal ‘Cureus’
- Latchupatula L, Benayon M, Yang L, Ganame J, Tandon V. COVID-19 mRNA Vaccination-Induced Myopericarditis in an Otherwise Healthy Young Male: An Evidence-Based Approach to Differentiating From Perimyocarditis. Cureus. 2024;16(5):e59999. Published 2024 May 9. doi:10.7759/cureus.59999
- ‘mRNA Vaccines Were Associated With Elevated Myocarditis/Pericarditis Risks Following Dose 2 in 12-39-Year-Olds’: Journal ‘American Journal of Epidemiology’
- Xu S, Sy LS, Han B, et al. A propensity score approach and a partitioned approach for the self-controlled case series design to evaluate safety of a 2-dose vaccine series: application to myocarditis/pericarditis following mRNA COVID-19 vaccination. Am J Epidemiol. 2025;194(1):254-266. doi:10.1093/aje/kwae141
- ‘Biopsy-Proven Inflammatory Dilated Cardiomyopathy Following Heterologous Mrna-1273 Third-Dose Immunization’: Journal ‘ESC Heart Failure’
- Hashimoto K, Yamamoto H, Ikeda Y, Isogai J, Hashimoto T. A case of biopsy-proven inflammatory dilated cardiomyopathy following heterologous mRNA-1273 third-dose immunization. ESC Heart Fail. 2024;11(6):4442-4449. doi:10.1002/ehf2.14924
- ‘Unilateral Combined Central Retinal Vein Occlusion, Incomplete Central Retinal Artery Occlusion, and Papillitis Following a Third Dose of COVID-19 Vaccination’: Journal ‘Frontiers in Ophthalmology’
- Furukawa A, Suzuki Y, Nozuki N, et al. Case report: A case of unilateral combined central retinal vein occlusion, incomplete central retinal artery occlusion, and papillitis following a third dose of COVID-19 vaccination. Front Ophthalmol (Lausanne). 2024;4:1352962. Published 2024 Feb 23. doi:10.3389/fopht.2024.1352962
- ‘Persistently High Platelet Factor 4 Levels in an Adolescent with Recurrent Late Thrombotic Complications after SARS-CoV-2 mRNA Vaccination’: Journal ‘Hematology Reports’
- Haga Y, Ohara A, Yakuwa T, et al. Persistently High Platelet Factor 4 Levels in an Adolescent with Recurrent Late Thrombotic Complications after SARS-CoV-2 mRNA Vaccination. Hematol Rep. 2024;16(3):504-511. Published 2024 Jul 29. doi:10.3390/hematolrep16030048
- ‘Onset of Leukocytoclastic Vasculitis Following COVID-19 Vaccination’: Journal ‘Rheumatology International’
- Miskovic R, Radovic S, Arandjelovic S, et al. Onset of leukocytoclastic vasculitis following covid-19 vaccination: case based comprehensive review. Rheumatol Int. 2024;44(11):2621-2635. doi:10.1007/s00296-024-05718-x
Considerando que las inyecciones basadas en ADN/ARN se han vinculado a graves complicaciones pulmonares y respiratorias, incluidos mayores riesgos de asma, síndrome de dificultad respiratoria aguda, exacerbación de la fibrosis pulmonar y trastornos respiratorios sistémicos, lo que pone de relieve importantes preocupaciones sobre su impacto en la salud respiratoria.
- Pulmón/Pulmonar/Respiratorio
- Children Who Receive COVID-19 Jab Nearly Twice as Likely to Develop Asthma: Journal ‘Infection’
- Yang CY, Shih YH, Lung CC. The association between COVID-19 vaccine/infection and new-onset asthma in children – based on the global TriNetX database. Infection. Published online June 21, 2024. doi:10.1007/s15010-024-02329-3
- Dorajoo SR, Tan HX, Teo CHD, et al. Nationwide safety surveillance of COVID-19 mRNA vaccines following primary series and first booster vaccination in Singapore. Vaccine X. 2023;15:100419. Published 2023 Dec 2. doi:10.1016/j.jvacx.2023.100419
- Tsumura K, Zaizen Y, Umemoto S, et al. Acute exacerbation of idiopathic pulmonary fibrosis after bivalent {tozinameran and famtozinameran} mRNA COVID-19 vaccination. Respir Med Case Rep. 2023;46:101960. Published 2023 Dec 9. doi:10.1016/j.rmcr.2023.101960
- Kawano F, Kato M, Tsuchida Y, Okawa J, Arimoto H. Acute Respiratory Distress Syndrome After Severe Acute Respiratory Syndrome Coronavirus (SARS-CoV-2) Vaccination: Findings From an Autopsy. Cureus. 2024;16(9):e70370. Published 2024 Sep 28. doi:10.7759/cureus.70370
- COVID-19 mRNA Jab to Fatal Pulmonary Hemorrhage 555 Days Post-Injection: ‘International Journal of Innovative Research in Medical Science’
- Hulscher N, McCullough PA. Delayed Fatal Pulmonary Hemorrhage Following COVID-19 Vaccination: Case Report, Batch Analysis, and Proposed Autopsy Checklist. Int J Innov Res Med Sci. 2025;10(02). doi:10.23958/ijirms/vol10-i02/2035.
- Children Who Receive COVID-19 Jab Nearly Twice as Likely to Develop Asthma: Journal ‘Infection’
Considerando que las inyecciones basadas en ADN/ARN se han visto implicadas en alteraciones endocrinas, incluida la aparición de niveles anormales de glucosa en sangre, lo que genera inquietudes sobre sus efectos sobre la salud metabólica y hormonal.
- Endocrino
- COVID-19 Vaccine ‘Appears to Be the Cause’ of ‘Abnormal Blood Glucose Level’: Letter in Journal ‘Advanced Biological Research’
- Mungmunpuntipantip R, Wiwanitkit V. COVID-19 Vaccination and Abnormal Blood Glucose Level. Adv Biomed Res. 2023;12:258. Published 2023 Nov 29. doi:10.4103/abr.abr_195_23
- COVID-19 Vaccine ‘Appears to Be the Cause’ of ‘Abnormal Blood Glucose Level’: Letter in Journal ‘Advanced Biological Research’
Teniendo en cuenta que las inyecciones basadas en ADN/ARN se han asociado con riesgos pediátricos significativos, incluida una mayor prevalencia de enfermedades en bebés, mayores tasas de enfermedades cardíacas, ansiedad, depresión y efectos neurológicos adversos en los niños, así como impactos alarmantes en el desarrollo de la descendencia, lo que genera profundas preocupaciones sobre su seguridad en poblaciones vulnerables.
- Pediatrico
- More Vaccinations Means ‘Exponentially’ More Disease in Infants: ‘International Journal of Vaccine Theory, Practice, and Research’
- Jablonowski K, Hooker B. Adverse Outcomes Are Increased with Exposure to Added Combinations of Infant Vaccines. Int J Vaccine Theory Pract Res. 2024;3(2):1103-1111. doi:10.56098/xfzkf650.
- Pradhan DD, Jena P, Misra S, Meher BK, Das L. Impact of Covid-19 on Psychosocial Well-Being of School-Going Children: A Cross-Sectional Study. Cureus. 2024;16(6):e62561. Published 2024 Jun 17. doi:10.7759/cureus.62561
- Andrews CD, Parker EP, Horne E, et al. OpenSAFELY: Effectiveness of COVID-19 vaccination in children and adolescents. medRxiv. Published May 20, 2024. doi:10.1101/2024.05.20.24306810.
- Donzelli A, Berrino F. Duration of SARS-CoV-2 Culturable Virus Shedding in Children by Vaccination Status. JAMA Pediatr. 2024;178(4):420-421. doi:10.1001/jamapediatrics.2023.6604
- Erdogan MA, Gurbuz O, Bozkurt MF, Erbas O. Prenatal Exposure to COVID-19 mRNA Vaccine BNT162b2 Induces Autism-Like Behaviors in Male Neonatal Rats: Insights into WNT and BDNF Signaling Perturbations. Neurochem Res. 2024;49(4):1034-1048. doi:10.1007/s11064-023-04089-2
- Tan JZ, Zhang DZ, Sundararaghavan S, et al. Chest pain attendances to a Paediatric Emergency Department pre- and post-COVID-19 vaccination. Transl Pediatr. 2023;12(11):2010-2019. doi:10.21037/tp-23-230
- Alghamdi Y, Abdulghani F, Huwait HF, Abdulghani M, Samarkandy SJ. An Unusual Presentation of Erythema Multiforme Following the Administration of Pfizer-BioNTech COVID-19 mRNA Vaccine in a Pediatric Patient. Cureus. 2024;16(4):e58450. Published 2024 Apr 17. doi:10.7759/cureus.58450
- CDC Study Confirms mRNA Jab-Injected Children Without Prior SARS-CoV-2 Infection 159% More Likely to Be Infected, 257% More Likely to Develop Symptomatic COVID-19 Compared to Unvaccinated Children Without Prior Infection: Oxford Academic’s Journal ‘Pediatric Infectious Diseases Society’
- Feldstein LR, Ruffin J, Wiegand R, et al. Protection From COVID-19 Vaccination and Prior SARS-CoV-2 Infection Among Children Aged 6 Months-4 Years, United States, September 2022-April 2023. J Pediatric Infect Dis Soc. 2025;14(1):piae121. doi:10.1093/jpids/piae121
- More Vaccinations Means ‘Exponentially’ More Disease in Infants: ‘International Journal of Vaccine Theory, Practice, and Research’
Las inyecciones basadas en ADN/ARN se han asociado con riesgos relacionados con el cáncer, incluida la presencia de proteínas pico o spike que pueden favorecer la supervivencia de las células cancerosas, la contaminación con secuencias SV40 vinculadas al cáncer, la estimulación del crecimiento tumoral y aumentos significativos en los diagnósticos y muertes por cáncer después de las campañas de vacunación masiva, lo que genera serias preocupaciones considerando su seguridad y sus efectos a largo plazo.
- Cáncer
- COVID Spike Protein—Present in Pfizer, Moderna mRNA Jabs—Helps Cancer Cells Survive Chemotherapy: Brown University Study Preprint in ‘BioRxiv’
- Zhang S, El-Deiry WS. SARS-CoV-2 spike S2 subunit inhibits p53 activation of p21(WAF1), TRAIL Death Receptor DR5, and MDM2 proteins in cancer cells. bioRxiv. Published online April 15, 2024. doi:10.1101/2024.04.12.589252.
- Raoult D. Confirmation of the presence of vaccine DNA in the Pfizer anti-COVID-19 vaccine. HAL Preprint. 2024. Available at: https://hal.science/hal-04778576v1.
- Rubio-Casillas A, Cowley D, Raszek M, Uversky VN, Redwan EM. Review: N1-methyl-pseudouridine (m1Ψ): Friend or foe of cancer? [published correction appears in Int J Biol Macromol. 2024 Jun;270(Pt 2):132447. doi: 10.1016/j.ijbiomac.2024.132447.]. Int J Biol Macromol. 2024;267(Pt 1):131427. doi:10.1016/j.ijbiomac.2024.131427
- Gibo M, Kojima S, Fujisawa A, Kikuchi T, Fukushima M. Increased Age-Adjusted Cancer Mortality After the Third mRNA-Lipid Nanoparticle Vaccine Dose During the COVID-19 Pandemic in Japan [retracted in: Cureus. 2024 Jun 26;16(6):r143. doi: 10.7759/cureus.r143.]. Cureus. 2024;16(4):e57860. Published 2024 Apr 8. doi:10.7759/cureus.57860
- Akkus E, Karaoglan B, Akyol C, et al. Types and Rates of COVID-19 Vaccination in Patients With Newly Diagnosed Microsatellite Stable and Instable Non-Metastatic Colon Cancer. Cureus. 2024;16(6):e61780. Published 2024 Jun 6. doi:10.7759/cureus.61780
- Nishizawa A, Kawakami M, Kitahara Y. Case report: A case of metastatic BRAFV600-mutated melanoma with heart failure treated with immune checkpoint inhibitors and BRAF/MEK inhibitors. Front Oncol. 2024;14:1366532. Published 2024 Mar 11. doi:10.3389/fonc.2024.1366532
- Valdes Angues R, Perea Bustos Y. SARS-CoV-2 Vaccination and the Multi-Hit Hypothesis of Oncogenesis. Cureus. 2023;15(12):e50703. Published 2023 Dec 17. doi:10.7759/cureus.50703
- Hitzenbichler F, Weber M, Salzberger B. Infection image: reoccurrence of Kaposi`s sarcoma after SARS-CoV-2 mRNA vaccination in an HIV-infected patient. Infection. 2024;52(1):283-284. doi:10.1007/s15010-023-02121-9
- Cervical Cancer Screening During COVID-19 Pandemic 3% Higher Compared to Pre-Pandemic Era: Journal ‘International Journal of General Medicine’
- Mitoma T, Maki J, Ooba H, Ogawa C, Masuyama H, Tabuchi T. Association of Regular Cervical Cancer Screening with Socioeconomic, COVID-19 Infection and Vaccine Status Among Japanese Population: Cohort Observational Study. Int J Gen Med. 2024;17:541-551. Published 2024 Feb 13. doi:10.2147/IJGM.S453675
- COVID Spike Protein—Present in Pfizer, Moderna mRNA Jabs—Helps Cancer Cells Survive Chemotherapy: Brown University Study Preprint in ‘BioRxiv’
Considerando que las inyecciones basadas en ADN/ARN se han relacionado con una variedad de efectos neurológicos graves, incluido el síndrome de Guillain-Barré, convulsiones, inflamación cerebral autoinmune, enfermedades neuromusculares y mayores riesgos de trastornos neurológicos como la parálisis de Bell y la encefalitis, lo que genera preocupaciones críticas sobre su impacto en el sistema nervioso y la salud general del cerebro.
- Efectos Neurologicos
- Guillain-Barré syndrome
- ‘Surge’ in Guillain-Barré Syndrome Cases Following COVID-19 Shot Rollout: ‘Scientific Reports’ Study Using WHO Data
- Jeong YD, Park S, Lee S, et al. Global burden of vaccine-associated Guillain-Barré syndrome over 170 countries from 1967 to 2023. Sci Rep. 2024;14(1):24561. Published 2024 Oct 19. doi:10.1038/s41598-024-74729-2
- Morciano C, Spila Alegiani S, Menniti Ippolito F, et al. Post-marketing active surveillance of Guillain Barré Syndrome following COVID-19 vaccination in persons aged ≥12 years in Italy: A multi-database self-controlled case series study. PLoS One. 2024;19(1):e0290879. Published 2024 Jan 19. doi:10.1371/journal.pone.0290879
- Kyo C, Kobayashi T, Iwama S, et al. A case of hypophysitis after COVID-19 vaccination with a detection of anti-pituitary antibody, with review of literature. Endocr J. 2024;71(8):799-807. doi:10.1507/endocrj.EJ24-0061
- Coviello A, Iacovazzo C, Vargas M, et al. A Death for Guillain-Barrè Syndrome After Receiving a COVID-19 Vaccine: A Case Report. Clin Med Insights Case Rep. 2024;17:11795476241274692. Published 2024 Oct 5. doi:10.1177/11795476241274692
- COVID-19 Jab Plays ‘Causal Role’ in Seizure Deaths: Journal ‘Academic Forensic Pathology’
- Prahlow JA. Deaths Related to New-Onset Seizures After Vaccination. Acad Forensic Pathol. Published online November 24, 2024. doi:10.1177/19253621241297029
- Di Tella M, Nahi YC, Paglia G, Geminiani GC. A Case Report of Autoimmune Encephalitis after Anti-SARS-CoV-2 Vaccination: The Role of Cognitive Impairments in the Diagnostic Process. Arch Clin Neuropsychol. 2024;39(6):775-781. doi:10.1093/arclin/acae031
- Prahlow JA. Deaths Related to New-Onset Seizures After Vaccination. Acad Forensic Pathol. Published online November 24, 2024. doi:10.1177/19253621241297029
- Autoimmune Brain Inflammation with ‘Psychiatric Manifestations’ Following Pfizer COVID-19 Injection: New Case Report in ‘Archives of Clinical Neuropsychology’
- Di Tella M, Nahi YC, Paglia G, Geminiani GC. A Case Report of Autoimmune Encephalitis after Anti-SARS-CoV-2 Vaccination: The Role of Cognitive Impairments in the Diagnostic Process. Arch Clin Neuropsychol. 2024;39(6):775-781. doi:10.1093/arclin/acae031
- Faksova K, Walsh D, Jiang Y, et al. COVID-19 vaccines and adverse events of special interest: A multinational Global Vaccine Data Network (GVDN) cohort study of 99 million vaccinated individuals. Vaccine. 2024;42(9):2200-2211. doi:10.1016/j.vaccine.2024.01.100
- Erdogan MA, Gurbuz O, Bozkurt MF, Erbas O. Prenatal Exposure to COVID-19 mRNA Vaccine BNT162b2 Induces Autism-Like Behaviors in Male Neonatal Rats: Insights into WNT and BDNF Signaling Perturbations. Neurochem Res. 2024;49(4):1034-1048. doi:10.1007/s11064-023-04089-2
- Al-Hawamdeh MI, Abu-Huwaij R, Astiti TA, Al-Debe’e AK, Abazeed OJ, Raees MA. Association between COVID-19 vaccines and development of chronic morbidities: a cross-sectional study in the Jordanian population. Curr Med Res Opin. 2024;40(3):537-543. doi:10.1080/03007995.2024.2303417
- Katz J, Sayman E, Gao H. The increased odds ratio for Bell’s palsy following COVID-19 infection or vaccination. Oral Dis. 2024;30(6):4054-4055. doi:10.1111/odi.14860
- Katz J, Sayman E, Gao H. The increased odds ratio for Bell’s palsy following COVID-19 infection or vaccination. Oral Dis. 2024;30(6):4054-4055. doi:10.1111/odi.14860
- Pilotto A, Catania M, Mattioli I, et al. Increased risk of functional neurological disorders following SARS-CoV-2 vaccination. Eur J Neurol. 2024;31(4):e16191. doi:10.1111/ene.16191
- Tayebi A, Samimisedeh P, Jafari Afshar E, et al. Neuromuscular diseases associated with COVID-19 vaccines: a systematic review and pooled analysis of 258 patients. BMC Neurol. 2023;23(1):437. Published 2023 Dec 11. doi:10.1186/s12883-023-03486-y
- Rogers C, Thorp JA, Cosgrove K, McCullough PA. COVID-19 Vaccines: A Risk Factor for Cerebral Thrombotic Syndromes. Int J Innov Res Med Sci. 2024;9(11). doi:10.23958/ijirms/vol09-i11/1982.
- Taylor ZC, Nunna RS, Tran A, et al. COVID-19 Vaccine Related Cervical Radiculitis and Parsonage-Turner Syndrome: Case Report and Review of the Literature. Turk Neurosurg. 2024;34(2):367-375. doi:10.5137/1019-5149.JTN.44533-23.2
- Sicard M, Shor N, Davy V, et al. Cerebellar encephalitis and peripheral neuropathy with an atypical clinical and neuroimaging signature following Covid-19 vaccine: a report of two cases. J Neurol. 2024;271(7):4680-4684. doi:10.1007/s00415-024-12390-5
- Roh JH, Jung I, Suh Y, Kim MH. A potential association between COVID-19 vaccination and development of Alzheimer’s disease. QJM. 2024;117(10):709-716. doi:10.1093/qjmed/hcae103
- ‘Surge’ in Guillain-Barré Syndrome Cases Following COVID-19 Shot Rollout: ‘Scientific Reports’ Study Using WHO Data
- Other nervous system effects
- Serious Cardiovascular, Respiratory, Immune, Digestive, Nervous System Conditions ‘Are Associated with Primary Vaccination Using COVID-19 mRNA Vaccines’: Peer-Reviewed Journal ‘Vaccine: X’
- Dorajoo SR, Tan HX, Teo CHD, et al. Nationwide safety surveillance of COVID-19 mRNA vaccines following primary series and first booster vaccination in Singapore. Vaccine X. 2023;15:100419. Published 2023 Dec 2. doi:10.1016/j.jvacx.2023.100419
- Serious Cardiovascular, Respiratory, Immune, Digestive, Nervous System Conditions ‘Are Associated with Primary Vaccination Using COVID-19 mRNA Vaccines’: Peer-Reviewed Journal ‘Vaccine: X’
- Guillain-Barré syndrome
Considerando que las inyecciones basadas en ADN/ARN se han asociado con trastornos del sistema linfático, incluidas linfadenopatía inducida por inyecciones y anomalías de los ganglios linfáticos axilares, lo que genera inquietudes sobre sus efectos sobre la función del sistema inmunológico y la salud linfática.
- Linfático
- ‘COVID-19 Vaccine-Associated Lymphadenopathy’: Journal ‘Le Infezioni in Medicina’
- Ciliberti V, Maffei E, Giudice V, Ciancia G, Zeppa P, Caputo A. COVID-19 vaccine-associated lymphadenopathy: a review. Infez Med. 2024;32(2):119-130. Published 2024 Jun 1. doi:10.53854/liim-3202-1
- Wilpert C, Wenkel E, Baltzer PAT, et al. Vaccine-associated axillary lymphadenopathy with a focus on COVID-19 vaccines. Impfassoziierte axilläre Lymphadenopathie mit Fokus auf COVID-19-Impfstoffe. Rofo. Published online June 21, 2024. doi:10.1055/a-2328-7536
- ‘COVID-19 Vaccination-Related [18F]Fdg-Avid Lymph Node in a Patient With Marginal Zone B-Cell Lymphoma’: Journal ‘Nuclear Medicine Review – Central & Eastern Europe’
- Ziolkowska P, Salyga A, Joks M, Ruchala M, Czepczynski R. COVID-19 vaccination-related [18F]FDG-avid lymph node in a patient with marginal zone B-cell lymphoma. Nucl Med Rev Cent East Eur. 2023;26(0):52-53. doi:10.5603/NMR.a2023.0004
- ‘COVID-19 Vaccine-Associated Lymphadenopathy’: Journal ‘Le Infezioni in Medicina’
Considerando que las inyecciones basadas en ADN/ARN se han vinculado a un aumento de los trastornos psicológicos, como la ansiedad, la depresión y la psicosis de nueva aparición, lo que agrava los problemas de salud mental ya acentuados por los confinamientos y los cierres de escuelas relacionados con la pandemia.
- Trastornos psicológicos
- More Than 4 in 10 Children Experience Anxiety, Depression from Mandated Pandemic Lockdowns, School Closures: Journal ‘Cureus’
- Pradhan DD, Jena P, Misra S, Meher BK, Das L. Impact of Covid-19 on Psychosocial Well-Being of School-Going Children: A Cross-Sectional Study. Cureus. 2024;16(6):e62561. Published 2024 Jun 17. doi:10.7759/cureus.62561
- Kim HJ, Kim MH, Choi MG, Chun EM. Psychiatric adverse events following COVID-19 vaccination: a population-based cohort study in Seoul, South Korea. Mol Psychiatry. 2024;29(11):3635-3643. doi:10.1038/s41380-024-02627-0
- Lazareva M, Renemane L, Vrublevska J, Rancans E. New-onset psychosis following COVID-19 vaccination: a systematic review. Front Psychiatry. 2024;15:1360338. Published 2024 Apr 12. doi:10.3389/fpsyt.2024.1360338
- ‘Potential Association Between COVID-19 Vaccination and Development of Alzheimer’s Disease’: Journal ‘QJM’
- Roh JH, Jung I, Suh Y, Kim MH. A potential association between COVID-19 vaccination and development of Alzheimer’s disease. QJM. 2024;117(10):709-716. doi:10.1093/qjmed/hcae103
- More Than 4 in 10 Children Experience Anxiety, Depression from Mandated Pandemic Lockdowns, School Closures: Journal ‘Cureus’
Considerando que las inyecciones basadas en ADN/ARN se han asociado con la aparición de enfermedades relacionadas con la diabetes, incluida la diabetes tipo 1 y tipo 2 de nueva aparición, la hiperglucemia transitoria y la diabetes insípida central, lo que genera inquietudes sobre su impacto en la salud metabólica y la regulación glucémica.
- Diabetes
- ‘COVID-19 RNA-Based Vaccines Might Cause New Onset’ Diabetes: ‘Journal of Medical Cases’
- Aburisheh KH, Enabi HMK, Alodah NA, et al. New Onset of Type 1 Diabetes Mellitus Post-COVID-19 Vaccine. J Med Cases. 2024;15(12):367-370. doi:10.14740/jmc4307
- Mazzeo P, Ceccato F, Manara R, Mazzon C, Barbot M. Transient Central Diabetes Insipidus (Arginine Vasopressin Deficiency) Following SARS-CoV-2 Vaccination: A Case Report and Literature Review. Endocr Metab Immune Disord Drug Targets. 2024;24(15):1856-1864. doi:10.2174/0118715303286560231124115052
- Takahashi H, Eguchi J, Watanabe M, Nakayama M, Wada J. Reduced Immunogenicity of COVID-19 Vaccine in Obese Patients with Type 2 Diabetes: A Cross-Sectional Study. Acta Med Okayama. 2024;78(2):185-191. doi:10.18926/AMO/66927
- Okon-Umoren A, Yaphe S, Smith A, Passalacqua KD, Budzynska K. Transient Hyperglycemia in a Patient With Type 2 Diabetes After COVID-19 Messenger RNA Vaccination: A Case Report. Cureus. 2024;16(7):e63983. Published 2024 Jul 6. doi:10.7759/cureus.63983\
- ‘Type 1 Diabetes Mellitus Following COVID-19 Vaccination’: Journal ‘Diabetology International’
- Mochizuki S, Miura J, Ucida K, et al. Type 1 diabetes mellitus following COVID-19 vaccination: a report of two cases and review of literature. Diabetol Int. 2024;15(3):577-582. Published 2024 Feb 29. doi:10.1007/s13340-024-00695-9
- ‘COVID-19 RNA-Based Vaccines Might Cause New Onset’ Diabetes: ‘Journal of Medical Cases’
Considerando que las inyecciones basadas en ADN/ARN se han vinculado a una incidencia preocupante de trastornos del sueño, lo que genera preocupaciones de salud adicionales sobre sus posibles efectos secundarios neurológicos y sistémicos.
- Trastornos del sueño
- ‘Concerning Incidence’ of Sleep Disorders Following COVID-19 Jab: Journal ‘Clinical and Experimental Vaccine Research’
- Al Katatbeh M, Al-Mashakbeh Y, Freihat H, et al. Incidence of narcolepsy symptoms after taking COVID-19 vaccines: a Jordanian cross-sectional study. Clin Exp Vaccine Res. 2024;13(3):218-224. doi:10.7774/cevr.2024.13.3.218
- Increased Psychiatric Disorders Following COVID Jab: Nature’s ‘Molecular Psychiatry’ Journal
- Kim HJ, Kim MH, Choi MG, Chun EM. Psychiatric adverse events following COVID-19 vaccination: a population-based cohort study in Seoul, South Korea. Mol Psychiatry. 2024;29(11):3635-3643. doi:10.1038/s41380-024-02627-0
- ‘Concerning Incidence’ of Sleep Disorders Following COVID-19 Jab: Journal ‘Clinical and Experimental Vaccine Research’
Considerando que las inyecciones basadas en ADN/ARN se han asociado con la pérdida del cabello, lo que genera inquietudes sobre posibles efectos secundarios dermatológicos y sistémicos.
- Pérdida del cabello
- Hair Loss Following COVID-19 Jab: Journal ‘Expert Opinion on Therapeutic Targets’
- Fu S, Song X. The clinical and immunological features of alopecia areata following SARS-CoV-2 infection or COVID-19 vaccines. Expert Opin Ther Targets. 2024;28(4):273-282. doi:10.1080/14728222.2024.2344696
- Hair Loss Following COVID-19 Jab: Journal ‘Expert Opinion on Therapeutic Targets’
Considerando que las inyecciones basadas en ADN/ARN se han asociado con complicaciones relacionadas con los nervios, incluida la parálisis motora del nervio radial y la neuralgia del trigémino, lo que genera inquietudes sobre sus efectos en el sistema nervioso periférico.
- Nervios
- ‘Radial Nerve Motor Palsy After COVID Vaccination’: ‘Journal of Family Medicine and Primary Care’ Case Report
- Radlberger RF, Trinka E, Leis S. Radial nerve motor palsy after COVID vaccination: A case report. J Family Med Prim Care. 2023;12(11):2967-2969. doi:10.4103/jfmpc.jfmpc_2437_22
- ‘Trigeminal Neuralgia Occurring After the Third Dose of Pfizer BioNTech COVID-19 Vaccine’: ‘Central European Journal of Immunology’
- Chrostowski K, Piasecki M, Bielewicz J. Trigeminal neuralgia occurring after the third dose of Pfizer BioNTech COVID-19 vaccine. Complication or coincidence? An illustrative case report and literature review. Cent Eur J Immunol. 2023;48(1):75-80. doi:10.5114/ceji.2023.125309
- ‘Radial Nerve Motor Palsy After COVID Vaccination’: ‘Journal of Family Medicine and Primary Care’ Case Report
Considerando que las inyecciones basadas en ADN/ARN se han relacionado con una amplia gama de eventos adversos dermatológicos y relacionados con la piel, incluida la aparición y exacerbación de psoriasis, vitíligo, dermatitis grave, alopecia, necrólisis epidérmica tóxica y otras afecciones cutáneas inflamatorias y autoinmunes, lo que genera serias preocupaciones sobre su impacto en la salud dermatológica.
- Piel/Dermatológico
- ‘New-Onset and Exacerbation of Psoriasis following COVID-19 Vaccination’: Indian Journal of Dermatology
- Arifin ANF, Hengky A, Widjaja M, Wijaya L. New-Onset and Exacerbation of Psoriasis following COVID-19 Vaccination: A Systematic Review of Case Reports and Case Series. Indian J Dermatol. 2023;68(6):724. doi:10.4103/ijd.ijd_833_22
- Al Hatmi I, Al Maqbali H, Al Waily A, Al Khalili A, Qureshi A. Pityriasis Rosea and Pityriasis Rosea-like Eruption Following COVID-19 Vaccinations: Case Series from Oman. Oman Med J. 2023;38(6):e579. Published 2023 Nov 30. doi:10.5001/omj.2024.01
- Nishizawa A, Kawakami M, Kitahara Y. Case report: A case of metastatic BRAFV600-mutated melanoma with heart failure treated with immune checkpoint inhibitors and BRAF/MEK inhibitors. Front Oncol. 2024;14:1366532. Published 2024 Mar 11. doi:10.3389/fonc.2024.1366532
- Tsai TF, Ng CY. COVID-19 vaccine-associated vitiligo: A cross-sectional study in a tertiary referral center and systematic review. J Dermatol. 2023;50(8):982-989. doi:10.1111/1346-8138.16799
- Katsube K, Nagai T, Watanabe T. Recurrent Skin Rash, Epigastralgia, and Arthralgia After SARS-CoV-2 mRNA Immunization and Breakthrough Infection. Gastroenterology. 2024;167(2):e5-e8. doi:10.1053/j.gastro.2023.12.024
- Patruno C, Fabbrocini G, Napolitano M. Severe symptomatic late onset dermographism after mRNA COVID-19 vaccine. Ital J Dermatol Venerol. 2024;159(1):60-61. doi:10.23736/S2784-8671.23.07454-6
- Huang T, Lv Y, Wang W, et al. Case Report: Fecal Microbiota Transplantation for the Treatment of Generalized Eczema Occurring After COVID-19 Vaccination. Clin Cosmet Investig Dermatol. 2024;17:229-235. Published 2024 Jan 26. doi:10.2147/CCID.S443542
- Zoli A, Peluso G, Grimaldi M, De Simone C, D’Agostino MA, Ortolan A. New onset of guttate psoriasis, Hallopeau’s continuous acrodermatitis, and psoriatic arthritis after COVID-19 vaccine. Scand J Rheumatol. 2024;53(5):361-362. doi:10.1080/03009742.2024.2316998
- Bolla E, Fragoulis GE, Iliopoulos A. New-Onset MDA-5 Dermatomyositis in a Patient Following COVID-19 Vaccination: A Case Report. Mediterr J Rheumatol. 2024;35(1):179-183. Published 2024 Mar 31. doi:10.31138/mjr.280124.nom
- Behera B, Thakur V, Sethy M, Ayyanar P, Garg S. COVID-19 Vaccine-Induced Erysipelas-Like Eruption. Indian J Dermatol. 2024;69(2):204. doi:10.4103/ijd.ijd_21_24
- Wali S, Gutte S, Pandey G, Kumar A, Gurjar M, Chahar JS. Toxic Epidermal Necrolysis After COVID-19 Vaccination: A Case Report and Review of Literature. J Acute Med. 2024;14(2):94-97. doi:10.6705/j.jacme.202406_14(2).0005
- Kim JS, Jeong CY, Lee GJ, Yeom SW, Nam KH. Risk of vitiligo in patients with SARS-CoV-2 vaccination or infection: a nationwide cohort study. Eur J Dermatol. 2024;34(2):150-157. doi:10.1684/ejd.2024.4646
- Zhu Y, Ouyang X, Zhang D, et al. Alopecia areata following COVID-19 vaccine: a systematic review. Eur J Med Res. 2024;29(1):356. Published 2024 Jul 5. doi:10.1186/s40001-024-01956-8
- Seol JE, Jang SH, Yun HW, Ahn SW, Kim H. A case of cutaneous sarcoidosis with pulmonary involvement after SARS-CoV-2 mRNA vaccination. JAAD Case Rep. 2024;50:47-50. Published 2024 May 28. doi:10.1016/j.jdcr.2024.05.019
- Daly TJ, Smith GT, McClain SA. Erythema multiforme-like papules after COVID vaccine administration. Dermatol Online J. 2024;30(3):10.5070/D330363868. Published 2024 Jun 15. doi:10.5070/D330363868
- Al Dhafiri M. COVID-19-Vaccination-Induced Localized Lichen Planus Successfully Treated With Phototherapy. Cureus. 2024;16(8):e66907. Published 2024 Aug 14. doi:10.7759/cureus.66907
- McIntyre E, Lamb P, Fung MA, Kiuru M, Chan LS. COVID-19 vaccination-linked granuloma annulare in two patients. Skin Health Dis. 2024;4(5):e412. Published 2024 Jul 24. doi:10.1002/ski2.412
- ‘COVID-19 and COVID-19 Vaccine-Related Skin Ulcerations in the Lower Extremities’: ‘International Journal of Lower Extremity Wounds’
- Beaineh P, El-Bsat A, Hafez B, et al. COVID-19 and COVID-19 Vaccine-Related Skin Ulcerations in the Lower Extremities: A Case Report and Literature Review. Int J Low Extrem Wounds. Published online October 29, 2024. doi:10.1177/15347346241275785
- ‘New-Onset and Exacerbation of Psoriasis following COVID-19 Vaccination’: Indian Journal of Dermatology
Considerando que las inyecciones basadas en ADN/ARN se han asociado con complicaciones oculares graves, que incluyen inflamación ocular, deficiencias en el suministro de sangre, ceguera, síndrome del punto blanco y púrpura de Henoch-Schönlein, lo que genera inquietudes sobre su seguridad e impacto en la visión y la salud ocular.
- Ojos/Ocular
- Eye Inflammation, Blood Supply Deficiency Following COVID Jab: ‘Korean Journal of Ophthalmology’
- Seo EJ, Jung MS, Lee K, Kim KT, Choi MY. Ischemic and Inflammatory Ocular Adverse Events Following Different Types of Vaccination for COVID-19 and Their Incidence Analysis. Korean J Ophthalmol. 2024;38(3):203-211. doi:10.3341/kjo.2023.0090
- Matri KE, Werda S, Chebbi Z, et al. Bilateral central retinal vein occlusion following COVID-19 vaccination: Cause or coincidence?. Eur J Ophthalmol. 2024;34(3):NP78-NP81. doi:10.1177/11206721241229109
- Matsuo T, Iguchi D. Rare Combination of Abducens Nerve Palsy and Optic Neuritis on the Same Side: Case Report and Review of 8 Patients in Literature. J Investig Med High Impact Case Rep. 2024;12:23247096231225873. doi:10.1177/23247096231225873
- Kashyap H, Manoharan A, Mahendradas P, Agarwal A, Majumder PD. A COVID-19 perspective of multiple evanescent white dot syndrome (MEWDS). Indian J Ophthalmol. 2024;72(5):620-625. doi:10.4103/IJO.IJO_2029_23
- Tetsumoto R, Matsumiya W, Sotani R, Kusuhara S, Nakamura M. Acute Noninfectious Anterior Ocular Inflammation Following Ranibizumab Biosimilar Intravitreal Injection in a Patient With Recent COVID-19 Vaccination. Cureus. 2024;16(5):e60356. Published 2024 May 15. doi:10.7759/cureus.60356
- Zeng Y, Du Z, Shao C, Zhao M. Comprehensive insights into COVID-19 vaccine-associated multiple evanescent white dot syndrome (MEWDS): A systematic analysis of reported cases. Hum Vaccin Immunother. 2024;20(1):2350812. doi:10.1080/21645515.2024.2350812
- ‘Henoch-Schönlein Purpura Following mRNA COVID-19 Vaccination’: Journal ‘Clinical & Experimental Vaccine Research’
- Lee MO, Yoo SJ. Henoch-Schönlein purpura following mRNA COVID-19 vaccination: a case report. Clin Exp Vaccine Res. 2024;13(2):166-170. doi:10.7774/cevr.2024.13.2.166
- Eye Inflammation, Blood Supply Deficiency Following COVID Jab: ‘Korean Journal of Ophthalmology’
Considerando que las inyecciones basadas en ADN/ARN se han vinculado a complicaciones ortopédicas y espinales, incluidas lesiones de la médula espinal, acumulación de sangre fuera de los vasos espinales, radiculitis cervical y síndrome de Parsonage-Turner, lo que genera preocupaciones importantes sobre su impacto en la salud musculoesquelética y neurológica.
- Ortopedia/Columna
- Woman Dies After Spinal Cord Injury, Heart Attack Following COVID-19 Vaccination: Acute Necrotizing Myelitis (‘Annals of Medicine & Surgery’ Case Study)
- Rezvani M, Mahmoodkhani M, Sourani A, et al. Treatment refractory acute necrotizing myelitis after COVID-19 vaccine injection: a case report. Ann Med Surg (Lond). 2024;86(2):1185-1190. Published 2024 Jan 3. doi:10.1097/MS9.0000000000001662
- Rezvani M, Sabouri M, Aminmansour B, et al. Spontaneous spinal epidural haematoma following COVID-19 vaccination: a case report. Ann Med Surg (Lond). 2023;86(1):612-619. Published 2023 Dec 8. doi:10.1097/MS9.0000000000001604
- ‘COVID-19 Vaccine Related Cervical Radiculitis and Parsonage-Turner Syndrome’: Journal ‘Turkish Neurosurgery’
- Taylor ZC, Nunna RS, Tran A, et al. COVID-19 Vaccine Related Cervical Radiculitis and Parsonage-Turner Syndrome: Case Report and Review of the Literature. Turk Neurosurg. 2024;34(2):367-375. doi:10.5137/1019-5149.JTN.44533-23.2
- Woman Dies After Spinal Cord Injury, Heart Attack Following COVID-19 Vaccination: Acute Necrotizing Myelitis (‘Annals of Medicine & Surgery’ Case Study)
Considerando que las inyecciones basadas en ADN/ARN se han asociado con un mayor riesgo de infecciones por herpes, incluida la reactivación del herpes zóster, herpes hemorrágico similar a la vasculitis y tasas de incidencia significativamente más altas en comparación con las personas no vacunadas, lo que genera inquietudes sobre sus efectos sobre la reactivación viral y la función del sistema inmunológico.
- Herpes
- Herpes After 3rd COVID-19 Vaccine in Cancer Patients: Journal ‘Clinical and Experimental Medicine’
- Nelli F, Fabbri A, Virtuoso A, et al. Herpes zoster after the third dose of SARS-CoV-2 mRNA-BNT162b2 vaccine in actively treated cancer patients: a prospective study. Clin Exp Med. 2024;24(1):13. Published 2024 Jan 20. doi:10.1007/s10238-023-01263-2
- Leeyaphan C, Jirawattanadon P, Bunyaratavej S, et al. Herpes Zoster after COVID-19 Infection or Vaccination: A Prospective Cohort Study in a Tertiary Dermatology Clinic. Dermatol Res Pract. 2023;2023:2206498. Published 2023 Dec 31. doi:10.1155/2023/2206498
- Cozma EC, Banciu LM, Soare C, Găman MA, Voiculescu VM. Vasculitis-Like Hemorrhagic Herpes Zoster and HIV Infections: An Intricate Association. Cureus. 2023;15(12):e50609. Published 2023 Dec 15. doi:10.7759/cureus.50609
- ‘Herpes Zoster Reactivation After COVID-19 Vaccine’: Journal ‘Anesthesiology & Pain Medicine’
- Chrona E, Tsoumani M, Batistaki C. Herpes Zoster Reactivation After COVID-19 Vaccine with Focus on Postherpetic Neuralgia Prevention: A Case Series. Anesth Pain Med. 2023;13(6):e131366. Published 2023 Dec 10. doi:10.5812/aapm-131366
- Herpes After 3rd COVID-19 Vaccine in Cancer Patients: Journal ‘Clinical and Experimental Medicine’
Considerando que las inyecciones basadas en ADN/ARN pueden influir en la patogénesis de la hepatitis autoinmune, lo que genera inquietudes sobre su potencial para desencadenar o exacerbar enfermedades hepáticas autoinmunes.
- Hepatitis
- ‘SARS-CoV-2 mRNA Vaccination May Influence the Pathogenesis’ of Autoimmune Hepatitis: Journal ‘Experimental & Therapeutic Medicine’
- Kuwano A, Nagasawa S, Koga Y, et al. Diagnostic features of autoimmune hepatitis in SARS‑CoV‑2‑vaccinated vs. unvaccinated individuals. Exp Ther Med. 2024;28(3):337. Published 2024 Jun 26. doi:10.3892/etm.2024.12626
- ‘SARS-CoV-2 mRNA Vaccination May Influence the Pathogenesis’ of Autoimmune Hepatitis: Journal ‘Experimental & Therapeutic Medicine’
Considerando que las inyecciones basadas en ADN/ARN se han asociado con reacciones de lepra inducidas, lo que genera inquietudes sobre su potencial para desencadenar o exacerbar enfermedades dermatológicas y sistémicas raras y graves.
- Lepra
- ‘COVID Vaccination Induced’ Leprosy Reactions: Journal ‘Indian Dermatology Online Journal’
- Kumari S, Verma GK, Negi AK, Sharma A. Lepra Reactions: Mimickers and Mirror to Unmask Complex Cases of Leprosy. Indian Dermatol Online J. 2023;15(3):500-503. Published 2023 Nov 24. doi:10.4103/idoj.idoj_294_23
- ‘COVID Vaccination Induced’ Leprosy Reactions: Journal ‘Indian Dermatology Online Journal’
Considerando que las inyecciones basadas en ADN/ARN se han relacionado con complicaciones hepáticas, incluido el síndrome de Budd-Chiari, lo que genera inquietudes sobre sus posibles efectos adversos sobre la salud del hígado y los sistemas vasculares.
- Hígado
- ‘Budd-Chiari syndrome after BNT162b2 mRNA vaccination’: Journal ‘European Journal of Hospital Pharmacy’
- Lipszyc L, Triquet L, Giguet B, Lambotte O, Babai S. Budd-Chiari syndrome after BNT162b2 mRNA vaccination: two case reports. Eur J Hosp Pharm. Published online July 22, 2024. doi:10.1136/ejhpharm-2023-003997
- ‘Budd-Chiari syndrome after BNT162b2 mRNA vaccination’: Journal ‘European Journal of Hospital Pharmacy’
Considerando que las inyecciones basadas en ADN/ARN pueden provocar el desprendimiento de nanopartículas lipídicas y proteínas pico o spike a través de transfusiones de sangre, leche materna, trasplante de órganos, exhalación y contacto de piel con piel, lo que genera serias preocupaciones sobre la exposición no intencionada y la transmisión a otras personas.
- Transmisión a otros
- COVID Vaccinated Could Shed Lipid Nanoparticles, Spike Protein Through Blood Transfusion, Breastmilk, Organ Transplantation, Exhalation, Skin-to-Skin Contact: New Preprint Study
- Halma MTJ, Rose J, McCullough PA. Inadvertent Exposure to Pharmacologically Designed Lipid Nanoparticles Via Bodily Fluids: Biologic Plausibility and Potential Consequences. Preprints. Published online February 22, 2024. doi:10.20944/preprints202402.1267.v1.
- ‘Indirect Exposure to COVID-19 Vaccinated Persons Was Significantly Associated with the Likelihood of the Onset of Menstrual Irregularities’: Journal ‘International Journal of Vaccine Theory, Practice, and Research’
- Peters SE, Newman J, Ray H, et al. Menstrual Abnormalities Strongly Associated with Proximity to COVID-19 Vaccinated Individuals. Int J Vaccine Theory Pract Res. 2024;3(2):1435-1452. doi:10.56098/tp99wn15.
- COVID Vaccinated Could Shed Lipid Nanoparticles, Spike Protein Through Blood Transfusion, Breastmilk, Organ Transplantation, Exhalation, Skin-to-Skin Contact: New Preprint Study
Considerando que las inyecciones basadas en ADN/ARN se han vinculado a importantes problemas reproductivos y de fertilidad, incluidas irregularidades menstruales, mayores tasas de aborto espontáneo, trastornos funcionales de la lactancia, riesgos de exposición fetal e impactos negativos en la calidad del esperma, lo que genera importantes preocupaciones sobre sus efectos en la salud reproductiva y los resultados..
- Reproducción/Fertilidad
- Menstrual
- ‘Many Women Experienced Various Menstrual Disturbances After Vaccination Against SARS-CoV-2’: International Journal of Fertility & Sterility
- Rahimi Mansour F, Keyvanfar A, Najafiarab H, Hooshmand Chayijan S, Farzaneh F, Mortezaei G. Menstrual Cycle Disturbances after COVID-19 Vaccination: A Cross-Sectional Study. Int J Fertil Steril. 2024;18(3):201-206. Published 2024 Jun 9. doi:10.22074/ijfs.2024.2016339.1579
- AlRawi HZ, AlQurashi A, AlDahan D, et al. Association between receiving Covid-19 vaccine and menstrual cycle patterns among childbearing women: A cross-sectional study. Health Sci Rep. 2024;7(5):e1934. Published 2024 May 9. doi:10.1002/hsr2.1934
- Latif R, Aldossary DA, Aljabari NA, et al. Prevalence of menstrual irregularities after coronavirus disease 2019 vaccination: A cross-sectional study in the Eastern Province, Saudi Arabia. J Family Community Med. 2024;31(1):71-78. doi:10.4103/jfcm.jfcm_115_23
- Aljehani AM, Banjar SA, Alawam HS, et al. The Relationship Between Menstrual Cycle Irregularities and COVID-19 Vaccination. Cureus. 2023;15(12):e49841. Published 2023 Dec 2. doi:10.7759/cureus.49841
- Tandon A, Kumar N, Aggarwal S, et al. Assessing Menstrual Changes Among Young Indian Females Post-SARS-CoV-2 Vaccination. Cureus. 2023;15(12):e50025. Published 2023 Dec 6. doi:10.7759/cureus.50025
- Arshad A, Usman M, Majeed Khan H, Rahman Z, Manzoor M, Gul B. Effect of COVID-19 vaccination on menstrual cycle irregularities among females: A cross-sectional study in Pakistan. Pak J Pharm Sci. 2024;37(1(Special)):215-222.
- Homam Safiah M, Kalalib Al Ashabi K, Khalayli N, Hodaifa Y, Kudsi M. The prevalence of menstrual changes in COVID-19 vaccinated women: A cross-sectional study. Prev Med Rep. 2024;44:102804. Published 2024 Jun 24. doi:10.1016/j.pmedr.2024.102804
- Al Shahrani A, Alhumaidan N, Alzelfawi L, et al. Prevalence of menstrual alterations following COVID-19 vaccination: systematic review & meta-analysis. BMC Womens Health. 2024;24(1):523. Published 2024 Sep 19. doi:10.1186/s12905-024-03349-9
- Peters SE, Newman J, Ray H, et al. Menstrual Abnormalities Strongly Associated with Proximity to COVID-19 Vaccinated Individuals. Int J Vaccine Theory Pract Res. 2024;3(2):1435-1452. doi:10.56098/tp99wn15.
- ‘Many Women Experienced Various Menstrual Disturbances After Vaccination Against SARS-CoV-2’: International Journal of Fertility & Sterility
- COVID-Jabbed Pregnant Women Have ‘Significantly Higher Rate’ of Miscarriage, Abnormal Amniotic Fluid and Placenta: Journal ‘Therapeutic Advances in Vaccines and Immunotherapy’
- Amer AA, Amer SA, Badokhon A, et al. Exploring COVID-19 vaccine adverse events among pregnant women: a cross-sectional study, 2022. Ther Adv Vaccines Immunother. 2024;12:25151355241285594. Published 2024 Oct 5. doi:10.1177/25151355241285594
- Thorp JA, Benavides A, Thorp MM, McDyer DC, Biss KO, Threet JA, McCullough PA. Are COVID-19 Vaccines in Pregnancy as Safe and Effective as the U.S. Government, Medical Organizations, and Pharmaceutical Industry Claim? Part I. Preprints. Published online June 29, 2024. doi:10.20944/preprints202406.2062.v1.
- Miller ND, Goren Gepstein N, Cohen D, et al. Does the SARS-CoV-2 mRNA vaccine and its serum IgG levels affect fertility treatments and obstetric outcomes? An observational cohort study. Clin Exp Med. 2024;24(1):81. Published 2024 Apr 23. doi:10.1007/s10238-024-01345-9
- Kang D, Choi A, Park S, Choe SA, Shin JY. Safety of COVID-19 Vaccination During Pregnancy and Lactation: A VigiBase Analysis. J Korean Med Sci. 2024;39(1):e3. Published 2024 Jan 8. doi:10.3346/jkms.2024.39.e3
- Zhong C, Cohen K, Lin X, Schiller E, Sharma S, Hanna N. COVID-19 Vaccine mRNA Biodistribution: Maternal and Fetal Exposure Risks. Am J Reprod Immunol. 2024;92(4):e13934. doi:10.1111/aji.13934
- Kang D, Choi A, Park S, Choe SA, Shin JY. Safety of COVID-19 Vaccination During Pregnancy and Lactation: A VigiBase Analysis. J Korean Med Sci. 2024;39(1):e3. Published 2024 Jan 8. doi:10.3346/jkms.2024.39.e3
- Trostle ME, Limaye MA, Avtushka V, Lighter JL, Penfield CA, Roman AS. COVID-19 vaccination in pregnancy: early experience from a single institution. Am J Obstet Gynecol MFM. 2021;3(6):100464. doi:10.1016/j.ajogmf.2021.100464
- Druškovič M, Lučovnik M, Mesarič VA, et al. Immune Response to SARS-CoV-2 in Vaccine-naive Pregnant Women: Assessment of IgG and IgA Antibody Profile at Delivery and 42 Days Postpartum. J Immunol. 2024;213(9):1371-1379. doi:10.4049/jimmunol.2400055
- Miller ND, Goren Gepstein N, Cohen D, et al. Does the SARS-CoV-2 mRNA vaccine and its serum IgG levels affect fertility treatments and obstetric outcomes? An observational cohort study. Clin Exp Med. 2024;24(1):81. Published 2024 Apr 23. doi:10.1007/s10238-024-01345-9
- Milostić-Srb A, Srb N, Talapko J, et al. The Effect of COVID-19 and COVID-19 Vaccination on Assisted Human Reproduction Outcomes: A Systematic Review and Meta-Analysis. Diseases. 2024;12(9):201. Published 2024 Sep 3. doi:10.3390/diseases12090201
- COVID-Jabbed Pregnant Women Have ‘Significantly Higher Rate’ of Miscarriage, Abnormal Amniotic Fluid and Placenta: Journal ‘Therapeutic Advances in Vaccines and Immunotherapy’
- Veteran OBGYN Calls on Lawyers to Represent Babies ‘Damaged’ by COVID-19 Jabs‘Autism-Like Behaviors’ in Offspring of COVID-Vaccinated Pregnant Rats: Journal ‘Neurochemical Research’
- Erdogan MA, Gurbuz O, Bozkurt MF, Erbas O. Prenatal Exposure to COVID-19 mRNA Vaccine BNT162b2 Induces Autism-Like Behaviors in Male Neonatal Rats: Insights into WNT and BDNF Signaling Perturbations. Neurochem Res. 2024;49(4):1034-1048. doi:10.1007/s11064-023-04089-2
- Zhong C, Cohen K, Lin X, Schiller E, Sharma S, Hanna N. COVID-19 Vaccine mRNA Biodistribution: Maternal and Fetal Exposure Risks. Am J Reprod Immunol. 2024;92(4):e13934. doi:10.1111/aji.13934
- Veteran OBGYN Calls on Lawyers to Represent Babies ‘Damaged’ by COVID-19 Jabs‘Autism-Like Behaviors’ in Offspring of COVID-Vaccinated Pregnant Rats: Journal ‘Neurochemical Research’
- Esperma
- ‘Concern’ COVID-19 Vaccine Negatively Affects Sperm Quality: Journal ‘Reproduction & Fertility’
- Mungmunpuntipantip R, Wiwanitkit V. COVID-19 vaccine and its effect on sperm. Reprod Fertil. 2023;4(1):e220098. Published 2023 Jan 25. doi:10.1530/RAF-22-0098
- ‘Concern’ COVID-19 Vaccine Negatively Affects Sperm Quality: Journal ‘Reproduction & Fertility’
- Menstrual
Las inyecciones basadas en ADN/ARN se han asociado con una amplia gama de trastornos del sistema inmunológico y autoinmunes, incluida la función inmunológica reducida, el lupus, la artritis reumatoide, la encefalitis autoinmune y la aparición o exacerbación de varias enfermedades autoinmunes, lo que genera preocupaciones críticas sobre su impacto en la regulación inmunológica y la salud sistémica.
- Sistema inmunológico/Autoinmune
- Repeated mRNA COVID Jabs Lead to Reduced Immune Function in Older Adults: Journal ‘Immunity & Ageing’
- Gelderloos AT, Verheul MK, Middelhof I, et al. Repeated COVID-19 mRNA vaccination results in IgG4 class switching and decreased NK cell activation by S1-specific antibodies in older adults. Immun Ageing. 2024;21(1):63. Published 2024 Sep 14. doi:10.1186/s12979-024-00466-9
- Vahedi M, Malek A, Saadat S, Tahghighi Sharabian F, Pourbadakhshan N. New Onset Systemic Lupus Erythematosus with Unusual Presentation and Multi-Organ Involvement after Covid-19 Vaccination in a Pediatric Patient: A Case Report. Iran J Kidney Dis. 2024;18(5):10.52547/rh64ar35. Published 2024 Oct 18. doi:10.52547/rh64ar35
- Fajloun Z, Tajer L, Khattar ZA, Sabatier JM. Unveiling the Role of SARS-CoV-2 or mRNA Vaccine Spike Protein in Macrophage Activation Syndrome (MAS). Infect Disord Drug Targets. Published online July 22, 2024. doi:10.2174/0118715265341206240722050403
- Tsai TF, Ng CY. COVID-19 vaccine-associated vitiligo: A cross-sectional study in a tertiary referral center and systematic review. J Dermatol. 2023;50(8):982-989. doi:10.1111/1346-8138.16799
- Di Tella M, Nahi YC, Paglia G, Geminiani GC. A Case Report of Autoimmune Encephalitis after Anti-SARS-CoV-2 Vaccination: The Role of Cognitive Impairments in the Diagnostic Process. Arch Clin Neuropsychol. 2024;39(6):775-781. doi:10.1093/arclin/acae031
- Al-Hawamdeh MI, Abu-Huwaij R, Astiti TA, Al-Debe’e AK, Abazeed OJ, Raees MA. Association between COVID-19 vaccines and development of chronic morbidities: a cross-sectional study in the Jordanian population. Curr Med Res Opin. 2024;40(3):537-543. doi:10.1080/03007995.2024.2303417
- Al-Hawamdeh MI, Abu-Huwaij R, Astiti TA, Al-Debe’e AK, Abazeed OJ, Raees MA. Association between COVID-19 vaccines and development of chronic morbidities: a cross-sectional study in the Jordanian population. Curr Med Res Opin. 2024;40(3):537-543. doi:10.1080/03007995.2024.2303417
- Kim S, Bea S, Choe SA, Choi NK, Shin JY. Autoimmune disorders reported following COVID-19 vaccination: A disproportionality analysis using the WHO database. Eur J Clin Pharmacol. 2024;80(3):445-453. doi:10.1007/s00228-023-03618-w
- Rahmatika AS, Murtiastutik D, Hidayati AN, et al. Unusual presentation of secondary syphilis mimicking erythema multiforme in HIV positive patient: a case report. Pan Afr Med J. 2023;46:55. Published 2023 Oct 16. doi:10.11604/pamj.2023.46.55.41497
- Ammer-Herrmenau C, Hamm J, Neesse A. Autoimmune pancreatitis-New evidence for clinical management strategies. United European Gastroenterol J. 2024;12(3):279-280. doi:10.1002/ueg2.12537
- Di Tella M, Nahi YC, Paglia G, Geminiani GC. A Case Report of Autoimmune Encephalitis after Anti-SARS-CoV-2 Vaccination: The Role of Cognitive Impairments in the Diagnostic Process. Arch Clin Neuropsychol. 2024;39(6):775-781. doi:10.1093/arclin/acae031
- Peraita-Adrados R, Bravo-Quelle N. Autoimmune encephalitis mediated by postvaccination and infection of SARS-CoV-2 in a patient with a narcolepsy type 1. Encefalitis autoinmune tras vacunación e infección por el SARS-CoV-2 en un paciente con narcolepsia de tipo 1. Rev Neurol. 2024;78(9):265-268. doi:10.33588/rn.7809.2023306
- Alghamdi Y, Abdulghani F, Huwait HF, Abdulghani M, Samarkandy SJ. An Unusual Presentation of Erythema Multiforme Following the Administration of Pfizer-BioNTech COVID-19 mRNA Vaccine in a Pediatric Patient. Cureus. 2024;16(4):e58450. Published 2024 Apr 17. doi:10.7759/cureus.58450
- Mullan G, Kinney M. Anti-LGI-1 Limbic Encephalitis and Autoimmune Epilepsy Following a Third Dose of COVID-19 Vaccination: A Case Report. Neurohospitalist. 2024;14(3):332-335. doi:10.1177/19418744241234100
- Miyagi Y, Kobayashi H, Umebayashi Y, et al. A Japanese case of VEXAS syndrome after COVID-19 vaccination: Comparison with previously reported cases. Mod Rheumatol Case Rep. 2025;9(1):218-223. doi:10.1093/mrcr/rxae054
- ‘Otitis Media with ANCA-Associated Vasculitis Following COVID-19 mRNA Vaccination’: Journal ‘American Journal of Case Reports’
- Yoshino Y, Yamanaka Y, Oda A. Otitis Media with ANCA-Associated Vasculitis Following COVID-19 mRNA Vaccination: A Case Report. Am J Case Rep. 2024;25:e945301. Published 2024 Sep 30. doi:10.12659/AJCR.945301
- Repeated mRNA COVID Jabs Lead to Reduced Immune Function in Older Adults: Journal ‘Immunity & Ageing’
Considerando que las inyecciones basadas en ADN/ARN se han asociado con una amplia gama de afecciones inflamatorias, incluidas enfermedades autoinflamatorias sistémicas, inflamación cerebral autoinmune, artritis psoriásica, artritis reumatoide y síndrome de activación de macrófagos, lo que genera preocupaciones importantes sobre su papel en el desencadenamiento y exacerbación de trastornos inflamatorios graves.
- Inflammación
- Systemic Autoinflammatory Disease Following COVID-19 Vaccination: Journal ‘Cureus’
- Nishioka H, Shirota S. Adult-Onset Still’s Disease After an mRNA COVID-19 Vaccine in an Older Woman. Cureus. 2024;16(1):e51540. Published 2024 Jan 2. doi:10.7759/cureus.51540
- Di Tella M, Nahi YC, Paglia G, Geminiani GC. A Case Report of Autoimmune Encephalitis after Anti-SARS-CoV-2 Vaccination: The Role of Cognitive Impairments in the Diagnostic Process. Arch Clin Neuropsychol. 2024;39(6):775-781. doi:10.1093/arclin/acae031
- Seo EJ, Jung MS, Lee K, Kim KT, Choi MY. Ischemic and Inflammatory Ocular Adverse Events Following Different Types of Vaccination for COVID-19 and Their Incidence Analysis. Korean J Ophthalmol. 2024;38(3):203-211. doi:10.3341/kjo.2023.0090
- Matsuo T, Iguchi D. Rare Combination of Abducens Nerve Palsy and Optic Neuritis on the Same Side: Case Report and Review of 8 Patients in Literature. J Investig Med High Impact Case Rep. 2024;12:23247096231225873. doi:10.1177/23247096231225873
- Al-Hawamdeh MI, Abu-Huwaij R, Astiti TA, Al-Debe’e AK, Abazeed OJ, Raees MA. Association between COVID-19 vaccines and development of chronic morbidities: a cross-sectional study in the Jordanian population. Curr Med Res Opin. 2024;40(3):537-543. doi:10.1080/03007995.2024.2303417
- Yeh YT, Tsai TF. Drug- or Vaccine-Induced/Aggravated Psoriatic Arthritis: A Systematic Review. Dermatol Ther (Heidelb). 2024;14(1):59-81. doi:10.1007/s13555-023-01082-z
- Al-Hawamdeh MI, Abu-Huwaij R, Astiti TA, Al-Debe’e AK, Abazeed OJ, Raees MA. Association between COVID-19 vaccines and development of chronic morbidities: a cross-sectional study in the Jordanian population. Curr Med Res Opin. 2024;40(3):537-543. doi:10.1080/03007995.2024.2303417
- Sousa M, Gersão S, Sousa HB. New-Onset Psoriatic Arthritis Following COVID-19 mRNA Vaccination in a Psoriatic Patient Under Anti-tumor Necrosis Factor Alpha Biologic Treatment: What Now?. Cureus. 2023;15(12):e50723. Published 2023 Dec 18. doi:10.7759/cureus.50723
- Almouslem A, Al Lawati T, Al Shirawi A, Al Amri U. New-onset Seropositive Rheumatoid Arthritis Post-mRNA COVID-19 Vaccine: A Case Report. Oman Med J. 2024;39(4):e597. Published 2024 Jan 31. doi:10.5001/omj.2024.06
- Yoshino Y, Tsuboi K. Eosinophilic Gastroenteritis Following Covid-19 MRNA Vaccination. Eur J Case Rep Intern Med. 2024;11(3):004316. Published 2024 Feb 12. doi:10.12890/2024_004316
- Narasimhan M, Ramakrishnan R, Varri DS, Sivasubramanian M. Lichen planus triggered by COVID-19 vaccination: A case series. J Family Med Prim Care. 2024;13(1):384-387. doi:10.4103/jfmpc.jfmpc_664_23
- Matsuda M, Funakubo Asanuma Y, Emoto K, et al. New-onset of rheumatic diseases following COVID-19 vaccination: the report of three cases and a literature review. Immunol Med. 2024;47(3):205-216. doi:10.1080/25785826.2024.2339542
- Kim J, Kwon HY, Ahn SJ. COVID-19 Vaccine-Associated Uveitis in Patients With a History of Uveitis. JAMA Ophthalmol. 2024;142(6):522-528. doi:10.1001/jamaophthalmol.2024.0973
- Mülkoğlu C, Tiftik T, Deniz AB, Taka İ, Genç H. Analysis of patients with adhesive capsulitis after COVID-19 vaccination: An observational study. Turk J Phys Med Rehabil. 2023;69(4):520-525. Published 2023 Oct 12. doi:10.5606/tftrd.2023.12660
- Niebrzydowski P, Kusiak-Kaczmarek M, Tomaszewski J, Gmiński M, Szalewska D. Case Report: The Rehabilitation of a Patient with Acute Transverse Myelitis after COVID-19 Vaccination. Clin Pract. 2024;14(3):1076-1084. Published 2024 Jun 6. doi:10.3390/clinpract14030085
- Saito S, Iijima M, Seki M, Shimomura A, Kitagawa K. Chronic Inflammatory Demyelinating Polyradiculoneuropathy with Diplopia Caused by an Alternative Coronavirus Disease 2019 Vaccine. Case Rep Neurol Med. 2024;2024:8584482. Published 2024 Jun 11. doi:10.1155/2024/8584482
- Fajloun Z, Tajer L, Khattar ZA, Sabatier JM. Unveiling the Role of SARS-CoV-2 or mRNA Vaccine Spike Protein in Macrophage Activation Syndrome (MAS). Infect Disord Drug Targets. Published online July 22, 2024. doi:10.2174/0118715265341206240722050403
- Pekdiker M, Ketenci S. ASIA syndrome after BNT162b2 vaccination: Is it a distinct rheumatoid arthritis phenotype?. Immunol Res. 2024;72(6):1424-1431. doi:10.1007/s12026-024-09540-2
- ‘Polymyalgia Rheumatica Following COVID-19 Vaccination’: Journal ‘Medicine’
- Irani L, Bou Karroum M, Chehab Y, Abi Saad N, Al Dailaty A, Husni R. Polymyalgia rheumatica following COVID-19 vaccination: Case series of 3 patients and literature review on polymyalgia rheumatica induced by various vaccines. Medicine (Baltimore). 2024;103(43):e40204. doi:10.1097/MD.0000000000040204
- Systemic Autoinflammatory Disease Following COVID-19 Vaccination: Journal ‘Cureus’
Considerndo que las inyecciones basadas en ADN/ARN se han vinculado al desarrollo de alergias a la carne en casi 2 de cada 10 receptores, lo que genera inquietudes sobre su potencial para inducir reacciones alérgicas graves y restricciones dietéticas.
- Alergias
- Meat Allergy in Nearly 2 in 10 COVID Jab Recipients: ‘The Journal of Allergy and Clinical Immunology: In Practice’
- Richards NE, Ailsworth SM, Workman LJ, et al. Mammalian Meat Allergy and IgE to Alpha-Gal in Central Virginia: Findings From a COVID-19 Vaccine and Patient Cohort. J Allergy Clin Immunol Pract. 2024;12(10):2817-2825.e2. doi:10.1016/j.jaip.2024.06.035
- Meat Allergy in Nearly 2 in 10 COVID Jab Recipients: ‘The Journal of Allergy and Clinical Immunology: In Practice’
Las inyecciones de ARNm autoamplificantes (samRNA) plantean riesgos importantes, incluido el potencial de inyectar virus vivos y autorreplicantes en los receptores debido a la contaminación, la producción de proteínas pico o spike aún más tóxicas en el cuerpo en comparación con las inyecciones de ARNm regulares y su clasificación como OGM por parte del gobierno australiano, lo que genera serias preocupaciones sobre su seguridad y supervisión regulatoria.
- Las inyecciones de ARNm autoamplificantes
- samRNA Vaccines Risk Injecting Live, Self-Replicating Viruses Into Recipients Due to Contamination: Journal ‘American Society for Microbiology’
- Hick TAH, Geertsema C, Nijland R, Pijlman GP. Packaging of alphavirus-based self-amplifying mRNA yields replication-competent virus through a mechanism of aberrant homologous RNA recombination. mBio. 2024;15(10):e0249424. doi:10.1128/mbio.02494-24
- 90% of Self-Amplifying mRNA Jab Recipients Suffer Adverse Event in Clinical Trials for ARCT-154: ‘Nature Communications’
- Hồ NT, Hughes SG, Ta VT, et al. Safety, immunogenicity and efficacy of the self-amplifying mRNA ARCT-154 COVID-19 vaccine: pooled phase 1, 2, 3a and 3b randomized, controlled trials. Nat Commun. 2024;15(1):4081. Published 2024 May 14. doi:10.1038/s41467-024-47905-1
- samRNA Vaccines Risk Injecting Live, Self-Replicating Viruses Into Recipients Due to Contamination: Journal ‘American Society for Microbiology’
Los tratamientos aprobados por la FDA, seguros, eficaces y fácilmente disponibles, como la budesonida, la ivermectina, la hidroxicloroquina y los anticuerpos monoclonales, han demostrado una eficacia significativa en el tratamiento temprano y tardío de COVID-19, y el testimonio de expertos y los estudios clínicos confirman su potencial para prevenir resultados graves y reducir la mortalidad, lo que genera inquietudes sobre la priorización de las inyecciones experimentales sobre estas terapias probadas.
- Tratamientos aprobados por la FDA, seguros, eficaces y fácilmente disponibles
- Budesonide
- Evidence – Budesonide Is Safe, Effective Early and Late Treatment for COVID-19
- Bartlett R, Fleetwood J. Evidence – Budesonide Is Safe, Effective Early and Late Treatment for COVID-19. G Med Sci. 2024;5(1):147-155. doi:10.46766/thegms.pubheal.24090601.
- ‘Ivermectin Is an Effective Medication’ for COVID-19: Double-Blind, Randomized Clinical Trial: ‘Jundishapur Journal of Health Sciences’
- Varnaseri M, Amini F, Jamshididan R, et al. Ivermectin as a Potential Addition to the Limited Anti-COVID-19 Arsenal: A Double-Blinded Clinical Trial. Jundishapur J Health Sci. 2024;16(2):e146703. doi:10.5812/jjhs-146703.
- Kerr L, Cadegiani FA, Baldi F, et al. Ivermectin Prophylaxis Used for COVID-19: A Citywide, Prospective, Observational Study of 223,128 Subjects Using Propensity Score Matching [published correction appears in Cureus. 2022 Mar 24;14(3):c61. doi: 10.7759/cureus.c61.]. Cureus. 2022;14(1):e21272. Published 2022 Jan 15. doi:10.7759/cureus.21272
- Hydroxychloroquine and Chloroquine Are Safe and Effective Against COVID-19: Double-Blind, Randomized, Placebo-Controlled Trial in Journal ‘PLOS Medicine’
- Schilling WHK, Mukaka M, Callery JJ, et al. Evaluation of hydroxychloroquine or chloroquine for the prevention of COVID-19 (COPCOV): A double-blind, randomised, placebo-controlled trial. PLoS Med. 2024;21(9):e1004428. Published 2024 Sep 12. doi:10.1371/journal.pmed.1004428
- ‘Potent neutralization by a receptor binding domain monoclonal antibody with broad specificity for SARS-CoV-2 JN.1 and other variants’: Journal ‘bioRxiv’
- Piepenbrink MS, Khalil AM, Chang A, et al. Potent neutralization by a receptor binding domain monoclonal antibody with broad specificity for SARS-CoV-2 JN.1 and other variants. Preprint. bioRxiv. 2024;2024.04.27.591446. Published 2024 Apr 29. doi:10.1101/2024.04.27.591446
- Evidence – Budesonide Is Safe, Effective Early and Late Treatment for COVID-19
- Other
- These 7 Natural Compounds Show ‘Potential Use in COVID-19 Treatment’: Journal ‘Dove Medical Press’
- Velásquez PA, Hernandez JC, Galeano E, Hincapié-García J, Rugeles MT, Zapata-Builes W. Effectiveness of Drug Repurposing and Natural Products Against SARS-CoV-2: A Comprehensive Review. Clin Pharmacol. 2024;16:1-25. Published 2024 Jan 4. doi:10.2147/CPAA.S429064
- These 7 Natural Compounds Show ‘Potential Use in COVID-19 Treatment’: Journal ‘Dove Medical Press’
- Budesonide
Teniendo en cuenta que las intervenciones farmacéuticas de ADN/ARN se han relacionado con graves efectos adversos en animales, incluido un shock anafiláctico en el 100% de los sujetos expuestos al ingrediente PEG, una mortalidad significativa en el ganado inyectado con inyecciones de ARN y comportamientos similares al autismo en las crías de ratas preñadas vacunadas, lo que genera serias preocupaciones sobre la seguridad de estas vacunas tanto para los animales como para los seres humanos.
Animales
- 100% of Animals Exposed to COVID Jab Ingredient ‘PEG’ Suffer Anaphylactic Shock Just 2 Minutes After Injection: Study in Journal ‘Vaccine: X’
- Barta BA, Radovits T, Dobos AB, et al. Comirnaty-induced cardiopulmonary distress and other symptoms of complement-mediated pseudo-anaphylaxis in a hyperimmune pig model: Causal role of anti-PEG antibodies. Vaccine X. 2024;19:100497. Published 2024 May 23. doi:10.1016/j.jvacx.2024.100497
- RNA Vaccine Injected Into U.S. Pork Livestock Kills 1 in 31 Pigs, Raising Meat Safety Concerns
- United States Department of Agriculture (USDA). Summary of Studies Supporting USDA Product Licensure: Swine Influenza Vaccine, N1 & N2, RNA Particle. USDA Vet Biologics. June 24, 2022. Establishment Number: 165A. Product Code: 19A5.R8.
- ‘Autism-Like Behaviors’ in Offspring of COVID-Vaccinated Pregnant Rats: Journal ‘Neurochemical Research’
- Erdogan MA, Gurbuz O, Bozkurt MF, Erbas O. Prenatal Exposure to COVID-19 mRNA Vaccine BNT162b2 Induces Autism-Like Behaviors in Male Neonatal Rats: Insights into WNT and BDNF Signaling Perturbations. Neurochem Res. 2024;49(4):1034-1048. doi:10.1007/s11064-023-04089-2
Considerando que no se han realizado estudios aleatorizados, doble ciego y controlados con placebo a largo plazo para evaluar la seguridad de las intervenciones farmacéuticas basadas en ADN/ARN en la producción agrícola, a pesar de la creciente evidencia de graves efectos adversos en humanos, animales y el medio ambiente, incluidos trastornos del sistema inmunológico, eventos cardiovasculares, complicaciones neurológicas, trastornos autoinmunes y riesgos de contaminación como la integración del SV40 y el ADN, lo que genera importantes preocupaciones sobre el impacto desconocido y potencialmente dañino de estas tecnologías en la seguridad alimentaria, el bienestar animal y la salud humana.
Ver más estudios sobre efectos adversos de la proteina pico o Spike de las inyecciones de ARNm aqui:
CATEGORIAS
A. General/Overview (32)
B. ACE2 (19)
C. Amyloid, prion-like properties (12)
D. Autoimmune (7)
E. Blood pressure/hypertension (2)
F. CD147 (13)
G. Cell membrane permeability, barrier dysfunction (13)
H. Cerebral, cerebrovascular, neurologic, blood-brain barrier, cognitive (24)
I. Clinical pathology (22)
J. Clotting, platelets, hemoglobin (30)
K. Cytokines, chemokines, interferon, interleukins (27)
L. Endothelial (25)
M. Gastrointestinal (8)
N. Immune dysfunction (5)
O. Macrophages , monocytes, neutrophils (28)
P. MAPK/NF-kB (10)
Q. Mast cells (3)
R. Microglia (6)
S. Microvascular (8)
T. MIS-C, pediatric (7)
U. Mitochondria / metabolism (9)
V. Myocarditis, cardiac, cardiomyopathy (22)
W. NLRP3 (15)
X. Ocular, ophthalmic, conjunctival (3)
Y. Other cell signaling (16)
Z. PASC, post COVID, long COVID (20)
AA. Pregnancy, fetal, placenta (7)
BB. Pulmonary, respiratory (28)
CC. Renin – Angiotensin-Aldosterone System (3)
DD. Senescence/aging (3)
EE. Stem cells (3)
FF. Syncytia / cell fusion (10)
GG. Therapeutics (37)
HH. Toll-like receptors (TLRs) (15)
Colabore por favor con nosotros para que podamos incluir mas información y llegar a más personas: contribución en mercado pago o paypal por única vez, Muchas Gracias!
Via PAYPAL: Euros o dólares click aqui
ARGENTINA 10.000$ar https://mpago.la/1srgnEY
5.000$ar https://mpago.la/1qzSyt9
1.000$ar https://mpago.la/1Q1NEKM
Solicite nuestro CBU contactenos